NHC稳定的双配位单金属和双金属Cu(I)配合物:配体定向发射和溶剂变色
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
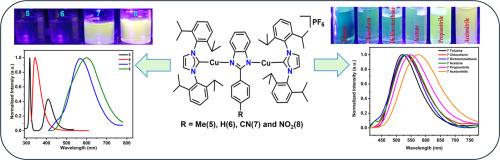
本文研究了由n -杂环羰基(NHC=1,3-二-(2,6-二异丙基苯基)咪唑-2-酰基)和取代的2-苯基苯并咪唑(PhBz)配体稳定的双配位单金属和双金属Cu(I)配合物的合成和光物理性质。合成了单金属配合物[Cu(NHC)(MePhBz)]PF6(1)、[Cu(NHC)(PhBz)]PF6(2)、[Cu(NHC)(CNPhBz)]PF6(3)、[Cu(NHC)(NO2PhBz)]PF6(4)和双金属配合物[(Cu(NHC)))2(MePhBz)]PF6(5)、[(Cu(NHC))2(PhBz)]PF6(6)、[(Cu(NHC))2(CNPhBz)]PF6(7)和[(Cu(NHC))2(NO2PhBz)]PF6(8)并对其进行了表征。据我们所知,这是双金属线性双配位Cu(I)配合物的首次报道。这些配合物在紫外可见范围内显示可调谐的发射,具有纳秒寿命(0.28-2.70 ns)。在PhBz配体框架上引入氰基(CN)和硝基(NO2)等吸电子取代基,通过稳定LUMO来调节电子结构,导致HOMO-LUMO能隙逐渐缩小,吸收/发射谱发生红移。此外,配合物3、4、7和8在不同极性的溶剂中表现出积极的溶剂致变色行为,其发射位移为1209至2661 cm−1。随着溶剂极性的增加,所观察到的色移归因于溶剂诱导的HOMO-LUMO能隙的减小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
NHC stabilized two-coordinated mono- and bimetallic Cu(I) complexes: Ligand-directed emission and solvatochromism
This study presents the synthesis and photophysical investigation of two-coordinated mono- and bimetallic Cu(I) complexes stabilized by sterically demanding N-heterocyclic carbene (NHC=1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene) and substituted 2-phenylbenzimidazole (PhBz) ligands. Monometallic complexes, [Cu(NHC)(MePhBz)]PF6 (1), [Cu(NHC)(PhBz)]PF6 (2), [Cu(NHC)(CNPhBz)]PF6 (3), [Cu(NHC)(NO2PhBz)]PF6 (4), and bimetallic complexes [(Cu(NHC))2(MePhBz)]PF6 (5), [(Cu(NHC))2(PhBz)]PF6 (6), [(Cu(NHC))2(CNPhBz)]PF6 (7) and [(Cu(NHC))2(NO2PhBz)]PF6 (8) were synthesized and thoroughly characterized. To the best of our knowledge, this constitutes the first report of bimetallic linear two-coordinated Cu(I) complexes. These complexes display tunable emission across the UV–visible range, with nanosecond lifetimes (0.28–2.70 ns). Introduction of electron-withdrawing substituents such as cyano (CN) and nitro (NO2) groups on the PhBz ligand framework modulates the electronic structure by stabilizing the LUMO, resulting in progressively narrowed HOMO–LUMO energy gaps and red-shifted absorption/emission profiles. Additionally, complexes 3, 4, 7 and 8 exhibit positive solvatochromic behaviour across solvents of varying polarity with emission shifts of 1209 to 2661 cm−1. The observed bathochromic shift with increasing solvent polarity is attributed to a solvent-induced decrease in the HOMO–LUMO energy gap.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


