基于8-羟基喹啉的六种新型双核钙(II)配合物抗癌作用的研究
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
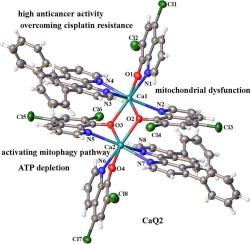
本研究报道了六种新型双核钙(II)配合物的合成和抗肿瘤评价,其通式为[Ca2(μ2-O)2(QMx)4(QHy)2],编号为CaQ1 ~ CaQ6。这些配合物包含各种去质子化的8-羟基喹啉配体(H-QM1−H-QM4)和1,10-菲罗啉衍生物(QH2),由Ca(NO3)2·4H2O合成。具体组成如下:CaQ1: H-QM1 = 5,7-二溴-8-羟基喹啉(x = 1), QH1 =邻苯二酚;CaQ2: H-QM2 = 5,7-二氯-8-喹啉(x = 2), QH1 =邻苯二酚;CaQ3: H-QM3 = 5,7-二碘-8-羟基喹啉(x = 3), QH2 = 1,10-菲罗啉;CaQ4: H-QM2 = 5,7-二氯-8-喹啉(x = 2), QH2 = 1,10-菲罗啉;CaQ5: H-QM4 = clioquinol (x = 4), QH2 = 1,10-菲罗啉;CaQ6: H-QM1 = 5,7-二溴-8-羟基喹啉(x = 1), QH2 = 1,10-菲罗啉。使用细胞计数试剂盒-8法评估细胞毒性,结果显示,与非致瘤性HL-7702肝细胞和亲代SK-OV-3卵巢癌细胞相比,CaQ1-CaQ6对顺铂耐药的SK-OV-3/DDP卵巢癌细胞(顺- sk3)具有更高的选择性。值得注意的是,含有活性H-QM1、H-QM2和QH1配体的CaQ1和CaQ2对顺式sk3细胞的IC50值分别为3.59±0.67 μM和2.73±0.25 μM,显示出最强的细胞毒性。CaQ1和CaQ2诱导的细胞凋亡是通过线粒体自噬激活和三磷酸腺苷耗散介导的,CaQ2比CaQ1更有效,可能是由于CaQ2复合体中存在QM2和QH1配体。综上所述,这些发现表明合成的8-羟基喹啉双核钙(II)-1,10-菲罗啉配合物(CaQ1−CaQ6)是很有前途的Ca(II)基抗癌药物候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development of six novel dinuclear calcium(II) complexes based on 8-hydroxyquinoline as anticancer agents
This study reports the synthesis and antitumor evaluation of six novel dinuclear calcium(II) complexes with the general formula [Ca2(μ2-O)2(QMx)4(QHy)2], designated as CaQ1 through CaQ6. These complexes incorporate various deprotonated 8-hydroxyquinoline ligands (H-QM1−H-QM4) and 1,10-phenanthroline derivatives (QH2), synthesized using Ca(NO3)2·4H2O. The specific compositions are as follows: CaQ1: H-QM1 = 5,7-dibromo-8-hydroxyquinoline (x = 1), QH1 = bathophenanthroline; CaQ2: H-QM2 = 5,7-dichloro-8-quinolinol (x = 2), QH1 = bathophenanthroline; CaQ3: H-QM3 = 5,7-diiodo-8-hydroxyquinoline (x = 3), QH2 = 1,10-phenanthroline; CaQ4: H-QM2 = 5,7-dichloro-8-quinolinol (x = 2), QH2 = 1,10-phenanthroline; CaQ5: H-QM4 = clioquinol (x = 4), QH2 = 1,10-phenanthroline; CaQ6: H-QM1 = 5,7-dibromo-8-hydroxyquinoline (x = 1), QH2 = 1,10-phenanthroline. Cytotoxicity was assessed using the cell counting kit-8 assay, and the results showed that CaQ1–CaQ6 exhibited greater selectivity toward cisplatin-resistant SK-OV-3/DDP ovarian (cis-SK3) cancer cells compared to both nontumorigenic HL-7702 liver cells and parental SK-OV-3 ovarian cancer cells. Notably, CaQ1 and CaQ2, which contain the active H-QM1, H-QM2, and QH1 ligands, showed the most potent cytotoxicity, with IC50 values of 3.59 ± 0.67 μM and 2.73 ± 0.25 μM, respectively, against cis-SK3 cells. Apoptosis induced by CaQ1 and CaQ2 was mediated by mitophagy activation and adenosine triphosphate depletion, with CaQ2 being more effective than CaQ1—likely due to the presence of QM2 and QH1 ligands in the CaQ2 complex. In conclusion, these findings suggest that the synthesized 8-hydroxyquinoline-based binuclear calcium(II)-1,10-phenanthroline complexes (CaQ1−CaQ6) represent promising Ca(II)-based anticancer drug candidates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


