一类新型青霉素结合蛋白2a抑制剂- 4h -4- 1衍生物处理微生物耐药性
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
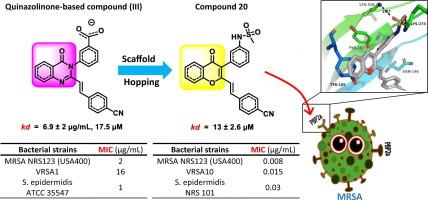
随着抗生素耐药性的持续上升和对几乎所有一线抗生素的敏感性降低,迫切需要开发新的、有效的和安全的替代品。在这项研究中,利用支架跳跃策略开发了一类新的青霉素结合蛋白2a (PBP2a)抑制剂,以4h - chromen4 -one核心结构为中心。这些新设计的化合物对耐甲氧西林金黄色葡萄球菌(MRSA)和其他耐药革兰氏阳性病原体具有很强的抗菌作用。值得注意的是,化合物16和18-20对葡萄球菌表现出显著的抑制作用,最低抑制浓度(mic)在0.008 ~ 1 μg/mL之间,优于万古霉素和利奈唑胺等标准处理,以及参比化合物III。这些衍生物还对一系列临床相关的多重耐药革兰氏阳性细菌保持活性,并且在人类细胞测定中未显示可检测到的细胞毒性。此外,化合物19和20与β-内酰胺类抗生素共同施用时,对两种MRSA菌株表现出协同作用。最后,这些衍生物与PBP2a的变构位点表现出良好的结合亲和力,解离常数在13 ~ 23 μM之间,表明这类新型衍生物通过非共价结合PBP2a的变构位点抑制PBP2a作为主要靶点,从而破坏细胞壁交联,导致细胞死亡。因此,报道的基于4h -4- 1的抑制剂值得进一步研究,作为治疗葡萄球菌感染的潜在治疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tackling microbial resistance with 4H-chromen-4-one derivatives as a novel class of penicillin binding protein 2a inhibitors
With the continued upsurge of antibiotic resistance and reduced susceptibility to almost all frontline antibiotics, there is a pressing need for the development of new, effective, and safe alternatives. In this study, a scaffold-hopping strategy was utilized to develop a novel class of penicillin-binding protein 2a (PBP2a) inhibitors, centered around a 4H-chromen-4-one core structure. These newly designed compounds demonstrated strong antibacterial efficacy against methicillin-resistant Staphylococcus aureus (MRSA) and other drug-resistant gram-positive pathogens. Notably, compounds 16 and 18–20 exhibited significant potency against the tested Staphylococcal strains, with minimum inhibitory concentrations (MICs) ranging from 0.008 to 1 μg/mL, outperforming standard treatments such as vancomycin and linezolid, as well as the reference compound III. These derivatives also retained their activity against a range of clinically relevant multidrug-resistant gram-positive bacteria and showed no detectable cytotoxicity in human cell assays. Additionally, compounds 19 and 20 displayed synergistic effects when co-administered with β-lactam antibiotics against two MRSA strains. Finally, these derivatives exhibited excellent binding affinities to the allosteric site of PBP2a, with dissociation constants ranging from 13 to 23 μM, indicating that this novel class inhibits PBP2a as the primary target by binding non-covalently to its allosteric site, hence impairing cell-wall crosslinking, and resulting in cell death. Accordingly, the reported 4H-chromen-4-one-based class of inhibitors merit further investigation as potential therapeutic options for treatment of staphylococcal infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


