血小板COX和LOX酶通过RhoA信号调节淀粉样蛋白β分泌:对神经退行性疾病的影响
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
花生四烯酸通过环氧合酶(COX)和脂氧合酶(LOX)途径代谢是炎症、血管稳态和神经元信号传导的基础。在这里,我们研究了血小板表达的COX (PTGS1)和LOX (ALOX12)亚型在淀粉样蛋白-β (a β)分泌中的作用,这一过程与脑淀粉样血管病(CAA)和阿尔茨海默病(AD)的发病机制有关。利用生物信息学蛋白质相互作用图谱、途径富集分析和实验验证相结合的综合方法,我们确定了PTGS和ALOX亚型与细胞骨架重塑、线粒体功能和囊泡运输之间的广泛网络。功能富集指出PTGS1和ALOX12在血小板活化和分泌过程中的关键作用。体外研究表明,用TRAP-6刺激人血小板会引发a - β40分泌的强劲增加,而COX抑制或阻断RhoA(细胞骨架动力学的关键调节因子)会显著减弱a - β40分泌。这些发现表明血小板来源的Aβ释放是由COX/ lox依赖性信号通过RhoA驱动的。虽然我们的结果支持COX/LOX-RhoA轴,但我们认识到因果关系仍有待完全确定,ALOX12的作用需要进一步的实验验证。鉴于Aβ40在CAA中的血管沉积,我们的研究结果表明血小板是神经血管淀粉样变性的重要外周贡献者。因此,该研究应被视为假设生成,强调靶向血小板信号通路的治疗潜力,以减轻a β驱动的血管病理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
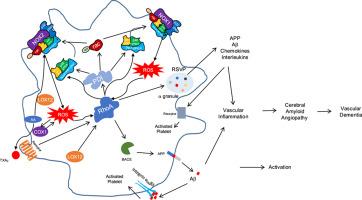
Platelet COX and LOX enzymes orchestrate amyloid-β secretion via RhoA signaling: Implications for neurodegenerative diseases
Arachidonic acid metabolism through cyclooxygenase (COX) and lipoxygenase (LOX) pathways is fundamental to inflammation, vascular homeostasis, and neuronal signaling. Here, we investigated the roles of platelet-expressed COX (PTGS1) and LOX (ALOX12) isoforms in amyloid-β (Aβ) secretion, a process implicated in the pathogenesis of cerebral amyloid angiopathy (CAA) and Alzheimer's disease (AD). Using an integrative approach combining bioinformatic protein–protein interaction mapping, pathway enrichment analysis, and experimental validation, we identified extensive networks linking PTGS and ALOX isoforms to cytoskeletal remodeling, mitochondrial function, and vesicle trafficking. Functional enrichment pointed to key roles for PTGS1 and ALOX12 in platelet activation and secretory processes. In vitro studies demonstrated that stimulation of human platelets with TRAP-6 triggered a robust increase in Aβ40 secretion, which was significantly attenuated by COX inhibition or blockade of RhoA, a critical regulator of cytoskeletal dynamics. These findings suggest that platelet-derived Aβ release is driven by COX/LOX-dependent signaling via RhoA. While our results support a COX/LOX-RhoA axis, we recognize that causality remains to be fully established, and the role of ALOX12 requires further experimental validation. Given the vascular deposition of Aβ40 in CAA, our results position platelets as important peripheral contributors to neurovascular amyloidosis. This study should therefore be viewed as hypothesis-generating, underscoring the therapeutic potential of targeting platelet signaling pathways to mitigate Aβ-driven vascular pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


