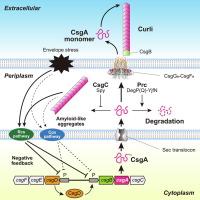
外质丝氨酸蛋白酶Prc负责大肠杆菌淀粉样蛋白亚基CsgA的降解和蛋白质停滞。
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
大肠杆菌在生物膜形成和寄主定植过程中在细胞外合成卷曲淀粉样纤维。蛋白质静止网络调节主要卷曲蛋白亚基CsgA,以防止细胞内淀粉样蛋白聚集,但其降解机制尚不明确。在这里,通过采用基因工程大肠杆菌、多拷贝抑制因子筛选和生化分析的综合研究,我们确定了质周丝氨酸蛋白酶Prc是CsgA降解的关键参与者。Prc通过内部裂解直接降解CsgA,与典型的尾部特异性蛋白酶不同。尽管细菌HtrA同源物DegP和DegQ在体外自杀激活剂YjfN存在下表现出有限的CsgA降解活性,但这些蛋白酶的删除并不影响体内天然CsgA降解。相反,Prc与质周伴侣CsgC协同,阻止了CsgA淀粉样聚集体的质周积累。此外,有效分泌和蛋白水解系统的损伤导致Rcs和Cpx双组分系统介导的csg操纵子表达减少。我们的研究结果揭示了大肠杆菌在蛋白质降解和转录调节水平上采用双层策略来阻止细胞内细胞外淀粉样蛋白的积累。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Periplasmic Serine Protease Prc is Responsible for Amyloid Subunit CsgA Degradation and Proteostasis in Escherichia coli
Escherichia coli synthesizes curli amyloid fibers extracellularly during biofilm formation and host colonization. The proteostasis network regulates the major curli subunit, CsgA, to prevent intracellular amyloid aggregation, yet the degradation mechanism remains elusive. Here, through a comprehensive investigation employing genetically engineered E. coli, multi-copy-suppressor screening, and biochemical analyses, we identify periplasmic serine protease Prc as a key player in CsgA degradation. Prc directly degrades CsgA through internal cleavage, differing from canonical tail-specific proteases. Although the bacterial HtrA homologs DegP and DegQ exhibit limited CsgA degradation activity in vitro in the presence of the suicide activator YjfN, deletion of these proteases did not affect native CsgA degradation in vivo. Instead, Prc, in coordination with the periplasmic chaperone CsgC, prevents the periplasmic accumulation of CsgA amyloid-like aggregates. Furthermore, impairment of efficient secretion and proteolytic systems leads to reduced csg operon expression mediated by the Rcs and Cpx two-component systems. Our findings reveal a dual-layered strategy employed by E. coli to prevent intracellular accumulation of extracellular amyloids at both protein degradation and transcriptional regulation levels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


