绿色负载的金纳米颗粒在锌铝层状双氧根上作为Suzuki-Miyaura偶联反应的有效纳米催化剂
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本研究阐述了以黄连木树皮提取物为天然还原剂和包衣剂,在锌铝层状双氧根表面修饰金纳米粒子的策略。锌铝LDH/Pistacia中植物分子的存在帮助创造了一种不使用有害物质将金离子转化为金颗粒的安全方法。为了研究Zn-Al LDH/Pistacia/Au NPs复合材料的结构,采用了一系列先进的技术,包括fesem -元素映射、EDX、TEM、ICP-OES和XRD。新制备的纳米催化剂在Suzuki-Miyaura偶联中进行了测试,以产生C-C键。Zn-Al LDH/Pistacia/Au NPs对各种芳基卤化物具有较高的催化活性。该催化剂具有良好的可回收性,即使经过8次反应也能保持原有的性能。热过滤法证实了催化剂的非均相性质,因为在反应过程中没有观察到浸出。本文章由计算机程序翻译,如有差异,请以英文原文为准。
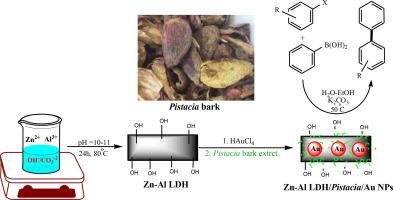
Green supported of gold nanoparticles over Zn–Al Layered Double Hydroxide as an effective nanocatalyst for Suzuki-Miyaura coupling reactions
This research explains a strategy for decoration of gold nanoparticles over the surface of Zn–Al Layered Double Hydroxide mediated by Pistacia bark extract as natural raducing and coating agent. The existence of phytomolecules in the Zn-Al LDH/Pistacia helped create a safe way to change gold ions into gold particles without using harmful substances. To study the structure of the Zn-Al LDH/Pistacia/Au NPs composite, a wide array of advanced techniques were applied, including FESEM-elemental mapping, EDX, TEM, ICP-OES, and XRD. The new produced nanocatalyst was tested in the Suzuki-Miyaura coupling to generate C–C bonds. The Zn-Al LDH/Pistacia/Au NPs illustrated a high level of catalytic activity with various aryl halides. Moreover, the catalyst shown good recyclability, keeping its performance even after 8 reaction runs. The heterogeneous nature of the catalyst was confirmed by a hot filtration method, as no leaching was observed during the reaction process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


