靶向蛋白降解中的分子胶和PROTACs:机制、进展和治疗潜力
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
靶向蛋白降解(TPD)是一种变革性的药物发现方法,可以调节以前认为“不可药物”的蛋白质。与传统抑制剂短暂抑制蛋白质活性不同,TPD利用泛素-蛋白酶体系统选择性地消除特定蛋白质,从而完全消除其活性。分子胶和靶向嵌合体(PROteolysis TArgeting Chimeras, PROTACs)是TPD中的两种主要方法,在机制和治疗应用上都有所不同。分子胶是一种低分子量的小分子,作为分子桥梁,促进目标蛋白和E3泛素连接酶之间的相互作用,从而实现降解,而不需要传统的结合袋。PROTACs是一种异功能小分子,它同时结合靶蛋白和E3连接酶,通过催化机制诱导选择性降解。这两种策略都极大地扩展了可药物蛋白质组,并为治疗干预提供了巨大的希望。本文综述了分子胶和PROTACs的比较概况,包括它们的作用机制、设计原理和治疗应用。我们强调了它们的物理化学性质、优点和局限性,以及最近的进展,这些进展正在推动新型降解剂的发现。通过临床进展和案例研究,我们研究了这些模式如何重塑药物发现并为各种疾病提供新的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
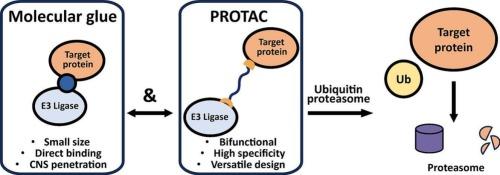
Molecular glues and PROTACs in targeted protein degradation: mechanisms, advances, and therapeutic potential
Targeted protein degradation (TPD) is a transformative approach to drug discovery that enables the modulation of proteins previously considered “undruggable.” Unlike traditional inhibitors, which transiently suppress protein activity, TPD harnesses the ubiquitin–proteasome system to selectively eliminate specific proteins and thereby fully abolish their activities. Two prominent approaches within TPD, Molecular Glues and PROteolysis TArgeting Chimeras (PROTACs), differ in both mechanism and therapeutic application.
Molecular Glues are small molecules with low molecular weight that act as a molecular bridge, facilitating interaction between a target protein and an E3 ubiquitin ligase to enable degradation without requiring classical binding pockets. PROTACs are heterobifunctional small molecules that simultaneously engage a target protein and an E3 ligase to induce selective degradation through a catalytic mechanism. Both strategies have vastly expanded the druggable proteome and hold great promise for therapeutic interventions.
This review provides a comparative overview of Molecular Glues and PROTACs, including their mechanisms, design principles, and therapeutic applications. We highlight their physicochemical properties, advantages, and limitations, as well as recent advances that are fueling the discovery of novel degraders. Through clinical advancements and case studies, we examine how these modalities are reshaping drug discovery and enabling new treatments for a variety of diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


