IFCT-0701 MAPS 3期试验中,MiR-193b-3p和miR-132-3p作为一线贝伐单抗加培美曲塞铂化疗胸膜间皮瘤患者生存的预后生物标志物
IF 5
2区 医学
Q2 Medicine
引用次数: 0
摘要
在间皮瘤阿瓦司汀顺铂培美曲塞研究(MAPS) 3期试验III期试验(NCT00651456)中,我们研究了血管生成相关microRNAs (miRNAs)是否能预测接受贝伐单抗加培美曲塞-铂化疗的胸膜间皮瘤(PM)患者的生存。在448例MAPS试验患者中的236例(50.8%)的FFPE样本中测量了12个mirna,归一化为RNU48。通过miRNA表达分析总生存期(OS)和无进展生存期(PFS),采用经临床协变量调整的单变量和多变量模型。内部验证是通过引导执行的。相互作用试验评估了每个miRNA相对于治疗组的预测价值。单因素和多因素分析均显示,低miR-193b-3p表达与PM患者较长的生存期相关(调整后HR = 0.87 [0.81-0.93], p < 0.001; bootstrap inclusion fraction [BIF]: 81.3%),两个治疗组一起分析。它还预测更长的PFS(调整后的HR = 0.91 [0.85-0.97], p = 0.0042)。相互作用试验显示,对于四种miRNAs (miR-155-5p、miR-29c-5p、miR-132-3p和miR-100-5p),较低的表达水平与贝伐单抗/顺铂/培美曲塞联合治疗的更高疗效相关。值得注意的是,治疗组与miR-132-3p表达之间的相互作用具有统计学意义(p = 0.004)。在IFCT-GFPC-0701 MAPS试验中,miR-193b-3p低表达表现出显著的独立预后价值,与更长的OS和PFS相关。此外,在贝伐单抗联合化疗组中,miR-155-5p、miR-29c-5p、miR-132-3p和miR-100-5p的低表达对改善生存率具有独立的预测价值。因此,对这四种mirna进行简单的qRT-PCR检测可能有助于识别最有可能从贝伐单抗中获益的PM患者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
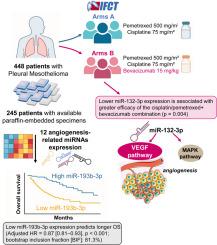
MiR-193b-3p and miR-132-3p as prognostic biomarkers of survival in pleural mesothelioma patients treated with first-line bevacizumab plus pemetrexed-platinum chemotherapy in the IFCT-0701 MAPS phase 3 trial
We investigated whether angiogenesis-related microRNAs (miRNAs) predict survival in patients with pleural mesothelioma (PM) treated with bevacizumab plus pemetrexed-platinum chemotherapy in the Mesothelioma Avastin Cisplatin Pemetrexed Study ('MAPS', NCT00651456) phase 3 trial phase III trial (NCT00651456). Twelve miRNAs were measured in FFPE samples from 236 of the 448 MAPS trial patients (50.8 %), normalized to RNU48. Overall survival (OS) and progression-free survival (PFS) were analyzed by miRNA expression using univariate and multivariate models adjusted for clinical covariates. Internal validation was performed by bootstrapping. Interaction tests assessed the predictive value of each miRNA with respect to treatment arm. Low miR-193b-3p expression was associated with longer OS in PM patients, as shown in both univariate and multivariate analyses (adjusted HR = 0.87 [0.81–0.93], p < 0.001; bootstrap inclusion fraction [BIF]: 81.3 %), with both treatment arms analyzed together. It also predicted longer PFS (adjusted HR = 0.91 [0.85–0.97], p = 0.0042). Interaction tests revealed that for four miRNAs (miR-155–5p, miR-29c-5p, miR-132–3p, and miR-100–5p), lower expression levels were associated with greater efficacy of the bevacizumab/cisplatin/pemetrexed combination. Notably, the interaction between treatment arms and miR-132–3p expression was statistically significant (p = 0.004). In the IFCT-GFPC-0701 MAPS trial, low miR-193b-3p expression demonstrated significant independent prognostic value, being associated with longer OS and PFS. Additionally, low expression of miR-155–5p, miR-29c-5p, miR-132–3p, and miR-100–5p showed independent predictive value for improved survival in the bevacizumab plus chemotherapy arm. Thus, a simple qRT-PCR assay of these four miRNAs may help identifying PM patients most likely to benefit from bevacizumab.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


