小胶质细胞来源的外泌体通过激活少突胶质细胞前体细胞的Nrf2信号通路调节髓鞘再生。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
脱髓鞘是多发性硬化症(MS)的一个显著特征,在多发性硬化症中,受损区域再生髓磷脂的能力受到限制。少突胶质前体细胞(OPCs)在这些区域积累,但难以成熟为少突胶质细胞(OLGs)。小胶质细胞也聚集在病变部位,但它们对OPCs分化的影响尚不清楚。在这里,我们发现从活化的小胶质细胞中提取的外泌体的表达谱中miR-155-5p显著升高。该miRNA与OPCs中转录因子Nrf2的3' UTR结合,抑制其分化。在铜铜酮诱导的小鼠脱髓鞘模型中,抑制小胶质细胞中的miR-155-5p可改善运动功能恢复,增加成熟少突胶质细胞数量,促进髓鞘再生。在这项研究中,我们强调了治疗脱髓鞘疾病的潜在新靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
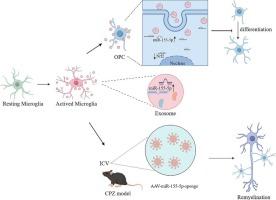
Microglia-derived exosomes modulate myelin regeneration via activating Nrf2 signaling pathway in oligodendrocyte precursor cells in MS
Demyelination is a prominent feature of multiple sclerosis (MS), where the ability of damaged areas to regenerate myelin is limited. Oligodendrocyte precursor cells (OPCs) accumulate in these areas but struggle to mature into oligodendrocytes (OLGs). Microglia also gather at the lesion site, but their impact on OPCs differentiation is not well understood. Here, we found that miR-155-5p was significantly elevated in the expression profile of exosomes extracted from activated microglia. This miRNA binds to the 3′ UTR of the transcription factor Nrf2 in OPCs, inhibiting their differentiation. In a mouse model of demyelination induced by cuprizone, inhibiting miR-155-5p in microglia led to improved motor function recovery, increased the number of mature oligodendrocytes and promoted remyelination. In this study, we highlight a potential new target for treating demyelinating diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


