薯蓣皂苷元降低突变α-syn聚集中β-薄片的含量:来自细胞内和细胞外神经元盐浓度构象动力学的见解
IF 3
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
帕金森病(PD)以α-syn蛋白及其突变形式(如A30P、A53T、E46K)聚集为标志,使α-syn蛋白更容易发生错误折叠和聚集,导致神经元细胞死亡。本研究探讨了薯蓣皂苷元(Diosgenin)在细胞外(0.145 M)和细胞内(0.015 M)盐浓度下抑制α-syn聚集的潜力。薯蓣皂苷元是一种通过ADMET预测鉴定的植物成分。分子动力学(MD)模拟表明,薯蓣皂苷元通过诱导构象变化,降低α-syn突变体聚集的关键因素β-sheet含量,从而稳定α-syn突变体。盐浓度影响结构动力学,高盐水平通常促进更紧密和稳定的构象。主成分分析(PCA)和自由能图进一步证实了薯蓣皂苷元结合α-syn突变体的稳定性增强。本研究结果提示,薯蓣皂苷元可能通过靶向α-syn突变体的聚集,并在细胞内和细胞外神经元盐浓度下降低α-syn突变体的β-片含量,作为一种有前景的减缓PD进展的治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
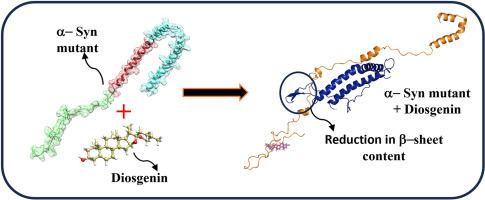
Diosgenin reduces β-sheet content in mutated α-syn aggregation: Insights from conformational dynamics at intracellular and extracellular neuronal salt concentrations
Parkinson's disease (PD) is marked by the aggregation of α-syn protein and its mutant forms, such as A30P, A53T, and E46K, which make the protein more prone to misfolding and aggregation, leading to neuronal cell death. This study explores the potential of Diosgenin, a phytoconstituent identified through ADMET predictions, to inhibit α-syn aggregation at both extracellular (0.145 M) and intracellular (0.015 M) salt concentrations. Molecular dynamics (MD) simulation revealed that Diosgenin stabilizes α-syn mutants by inducing conformational changes that reduce β-sheet content, a key factor in aggregation. The salt concentration influenced the structural dynamics, with higher salt levels generally promoting more compact and stable conformations. Principal component analysis (PCA) and free energy landscapes further confirmed the enhanced stability of the Diosgenin-bound α-syn mutants. The outcomes of the study suggest that Diosgenin could serve as a promising therapeutic agent for mitigating PD progression by targeting the aggregation of α-syn mutants and reducing its β-sheet content at intracellular and extracellular neuronal salt concentration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


