眼眶附近肿瘤放射治疗中光学结构运动的量化
IF 3.3
Q2 ONCOLOGY
引用次数: 0
摘要
背景与目的眼眶附近肿瘤放射治疗过程中光学结构的移动可能影响放射量,从而影响副作用的发生。因此,本研究的目的是量化放射治疗期间光学结构的运动。材料与方法回顾性分析20例脑肿瘤患者,在没有注视指导的情况下进行计划CT扫描(pCT)和5例重复CT扫描(reCTs)。双侧六个光学结构:晶状体、角膜、视网膜、泪腺、黄斑和视神经(ON)。联合国分为三个次区域。计算pCT和reCT之间的骰子相似系数(DSC)、绝对距离(AD)和3D中点差(ΔMP),计算90%患者覆盖95%体积的计划风险体积(PRV)边缘和各向同性扩张。对神经和鼻咽肿瘤进行了剂量-体积原理证明。结果角膜最高中位ΔMP为1.9 mm。对于ON亚区域,最高中位数和95百分位数ΔMP为近端眶内ON: 1.3 mm和3.1 mm。ON的中位AD最高:3.0 mm(负z方向)。睁眼状态导致ON、颅内ON和近端眶内ON的DSC较低,而近端眶内ON的DSC较高ΔMP。独立结构需要1-4毫米的各向同性膨胀,这取决于典型的运动范围。神经系统的剂量差异大于鼻咽部计划。结论观察到的光学结构运动提示临床应考虑prv切缘。在治疗过程中要求病人闭上眼睛可以减少他们的活动。本文章由计算机程序翻译,如有差异,请以英文原文为准。
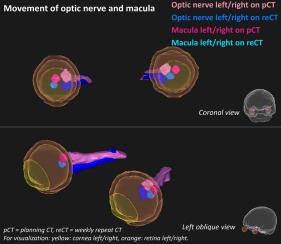
Quantifying movement of optical structures during radiotherapy treatment for tumors near the orbita
Background and purpose
Movement of optical structures during radiotherapy for tumors near the orbita might affect the amount of radiation given and consequently the risk for side effects. The aim of this study was therefore to quantify motion of optical structures during radiotherapy.
Materials and methods
Twenty brain tumor patients were retrospectively included, with planning computed tomography (CT)-scan (pCT) and five repeat-CT-scans (reCTs) without gazing instructions. Six optical structures were delineated bilaterally: lens, cornea, retina, lacrimal glands, macula, and optic nerves (ON). The ON was split in three subregions. The dice similarity coefficient (DSC), absolute distance (AD), and difference in 3D midpoint (ΔMP) were calculated between pCT and reCT. Planning risk volume (PRV)-margins and isotropic expansions to cover 95 % volume for 90 % patients were calculated. A dose-volume proof-of-principle was performed for a neurological and nasopharyngeal tumor.
Results
Highest median ΔMP was found for the cornea: 1.9 mm. For ON subregions, highest median and 95th-percentile ΔMP was found for proximal intra-orbital ON: 1.3 mm and 3.1 mm. ON showed highest median AD: 3.0 mm (negative Z-direction). Open eyelid status resulted in statistically significant lower DSC for ON, intra-cranial ON, and proximal intra-orbital ON, and higher ΔMP for proximal intra-orbital ON. 1–4 mm isotropic expansions were needed for separate structures, dependent on typical movement range. Higher dose-differences were found for the neurological than the nasopharyngeal plan.
Conclusions
The observed movement of optical structures indicated that a PRV-margin should be considered in clinical practice. Asking the patient to close their eyes during the treatment could decrease the movement.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physics and Imaging in Radiation Oncology
Physics and Astronomy-Radiation
CiteScore
5.30
自引率
18.90%
发文量
93
审稿时长
6 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


