母亲的分离破坏了成人应对行为的去肾上腺素能控制。
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
早期生活压力(ELS)深刻地影响着大脑,并与成年后的消极情感行为相关。蓝斑核(LC)是一种对压力有反应的脑干核,为大脑提供大部分去甲肾上腺素(NE),已知可调节负面情绪。采用重复母亲分离应激(MSS)方法,研究了ELS对成年期LC及应激相关行为的影响。我们在整个生命周期中进行了离体细胞附着电生理学研究,发现MSS显著增加了早期发育和成年期的LC放电,但在青春期前和青春期没有。接下来,我们研究了与LC功能相关的基因表达的潜在变化。在成年期,MSS降低了α - 2a肾上腺素能受体和多巴胺-羟化酶(NE合成所必需的酶)的mRNA水平。在行为水平上,MSS增加了趋近-回避探索性测试中的运动能力,增加了强迫游泳测试中的不动能力。虽然在无MSS和无MSS小鼠中,强迫游泳增加了神经元兴奋标志物LC cFos的表达,但在无MSS小鼠中,这种增加明显低于无MSS小鼠。我们进一步发现,MSS减少了LC细胞的数量,这可能是MSS和No MSS小鼠之间cFos诱导和基因表达差异的原因。最后,我们发现抑制No MSS小鼠的LC增加了静止时间,但不影响MSS的静止。相反,LC抑制使MSS小鼠攀爬时间增加。综上所述,本研究表明,MSS在整个生命周期中失调了LC- ne的活性,并破坏了LC对应激事件中应对策略的调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
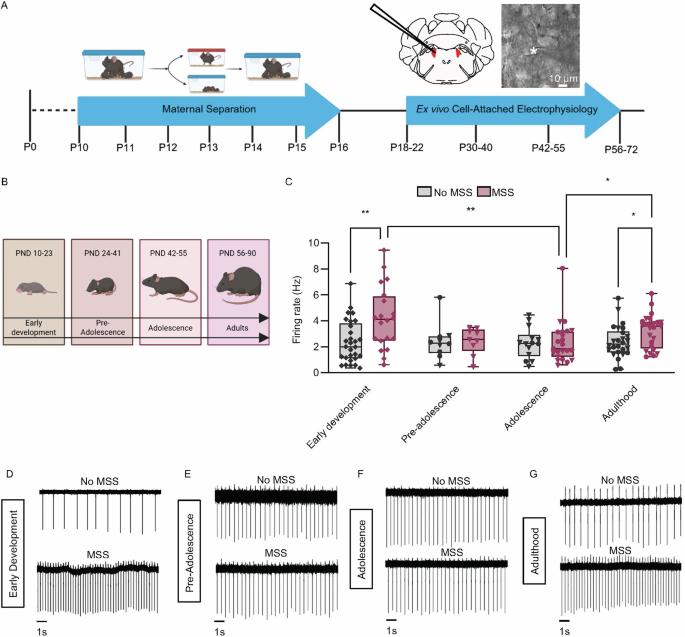
Maternal separation disrupts noradrenergic control of adult coping behaviors
Early life stress (ELS) profoundly impacts the brain and correlates with negative affective behaviors in adulthood. The locus coeruleus (LC), a stress-responsive brainstem nucleus that supplies most of the brain with norepinephrine (NE), is known to modulate negative affect. Using repeated maternal separation stress (MSS), we investigated the impact of ELS on the LC and stress-related behaviors in adulthood. We performed ex vivo cell-attached electrophysiology across the lifespan to reveal that MSS significantly increased LC firing during early development and adulthood but not in pre-adolescence and adolescence. We next examined potential changes in the expression of genes linked to LC function. In adulthood, MSS decreased mRNA levels for both the alpha-2A adrenergic receptor and dopamine beta-hydroxylase, the enzyme necessary for NE synthesis. At the behavioral level, MSS increased locomotion in approach-avoidance exploratory assays and increased immobility in the forced swim test. While forced swim increased LC cFos expression, a marker for neuronal excitation, in both No MSS and MSS mice, this increase was significantly lower in MSS mice than in No MSS controls. We further showed that MSS decreased the number of LC cells, possibly underlying the difference in cFos induction and gene expression between MSS and No MSS mice. Finally, we showed that inhibiting the LC in No MSS mice increased immobility time, but did not affect MSS immobility. Instead, LC inhibition in MSS mice increased climbing time. Together, this study demonstrates that MSS dysregulates LC-NE activity across the lifespan and disrupts LC regulation of coping strategies during stressful events.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


