精神分裂症患者的血液和神经元细胞外囊泡线粒体中断。
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
人类大脑的高能量需求需要强大的线粒体能量代谢,而线粒体功能障碍与包括精神分裂症谱系障碍(SSD)在内的神经精神疾病有关。然而,能够直接告知脑线粒体功能及其导致SSD的病因病理生理途径的体内评估仍然难以获得。我们假设SSD患者的系统和脑线粒体功能障碍可能通过血液和神经元细胞外囊泡(nev)中游离线粒体DNA (cf-mtDNA)水平升高来指示。我们还探讨了这些mtDNA标记物的升高是否与磁共振波谱(MRS)测量的脑代谢物有关。我们检测了58名SSD患者和33名健康对照者的血液cf-mtDNA,随后在一组患者和对照组中使用MRS评估nEV mtDNA和代谢物水平。我们发现,与对照组相比,SSD患者血液中的cf-mtDNA水平(p = 0.0002)和神经元ev (p = 0.003)均显著升高。这些mtDNA异常可以追溯到脑乳酸+水平,因此在SSD患者中,较高的血液和nEV mtDNA水平与前扣带皮层测量的较高乳酸+水平显著相关(r = 0.53, 0.53; p = 0.008, 0.03)。此外,较高的发育应激和创伤与SSD患者血液和神经元EVs中较高的cf-mtDNA水平显著相关(r = 0.29, 0.49; p = 0.01, 0.03)。总之,如果血液和神经元中基于ev的无细胞mtDNA得到复制和充分发育,可能为更直接地评估线粒体假说和精神分裂症异常生物能量学途径提供临床可及的生物标志物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
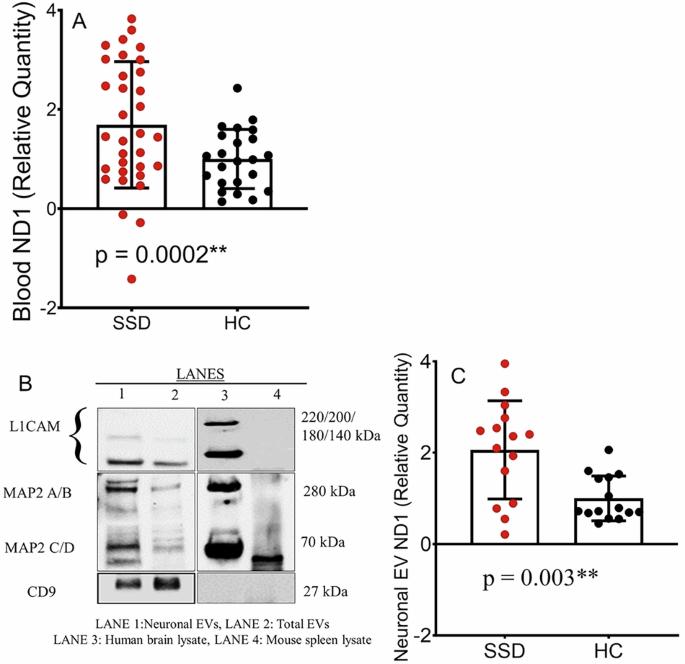
Blood and neuronal extracellular vesicle mitochondrial disruptions in schizophrenia
The high energy demand of the human brain obligates robust mitochondrial energy metabolism, while mitochondrial dysfunctions have been linked to neuropsychiatric disorders, including schizophrenia spectrum disorders (SSD). However, in vivo assessments that can directly inform brain mitochondrial functioning and its etiopathophysiological path to SSD remain difficult to obtain. We hypothesized that system and brain mitochondrial dysfunctions in SSD may be indexed by elevated cell-free mitochondrial DNA (cf-mtDNA) levels in the blood and in neuronal extracellular vesicles (nEVs). We also explored if these mtDNA marker elevations were associated with brain metabolites as measured by magnetic resonance spectroscopy (MRS). We examined blood cf-mtDNA in 58 SSD patients and 33 healthy controls, followed by assessing nEV mtDNA and metabolite levels using MRS in a subgroup of patients and controls. We found that people with SSD had significantly elevated cf-mtDNA levels in both the blood (p = 0.0002) and neuronal EVs (p = 0.003) compared to controls. These mtDNA abnormalities can be linked back to brain lactate+ levels such that higher blood and nEV mtDNA levels were significantly associated with higher lactate+ levels measured at the anterior cingulate cortex (r = 0.53, 0.53; p = 0.008, 0.03, respectively) in SSD patients. Furthermore, higher developmental stress and trauma were significantly associated with higher cf-mtDNA levels in both the blood and neuronal EVs in SSD patients (r = 0.29, 0.49; p = 0.01, 0.03, respectively). In conclusion, if replicated and fully developed, blood and neuronal EV-based cell-free mtDNA may provide a clinically accessible biomarker to more directly evaluate the mitochondrial hypothesis and the abnormal bioenergetics pathways in schizophrenia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


