METTL14启动m6A mRNA甲基化,促进STK11翻译,增加STK11活性,诱导乳腺癌抗her2治疗耐药。
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-08-28
DOI:10.1016/j.bbadis.2025.168029
引用次数: 0
摘要
对her2靶向治疗的耐药是治疗her2阳性乳腺癌患者的主要挑战。N(6)-甲基腺苷(m6A)修饰在肿瘤进展中起关键作用;然而,其在介导抗her2治疗耐药中的作用仍不明确。在曲妥珠单抗耐药her2阳性乳腺癌组织中,METTL14表达显著上调,并与曲妥珠单抗不良反应相关。此外,我们的数据表明转录因子RAD21通过结合METTL14的启动子区直接调节其表达。METTL14表达升高可增强对her2靶向治疗的耐药性,而METTL14敲低可恢复耐药乳腺癌细胞对曲妥珠单抗的敏感性。在机制上,METTL14促进STK11 mRNA的m6A甲基化,以m6A依赖的方式增加其稳定性,从而促进耐药性。综上所述,我们的研究结果定义了一个新的RAD21-METTL14-STK11轴,该轴驱动her2阳性乳腺癌的曲妥珠单抗耐药,并突出了克服治疗失败的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
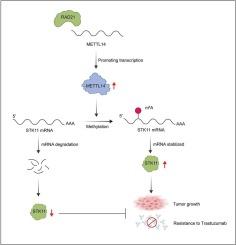
m6A mRNA methylation initiated by METTL14 promotes STK11 translation and increases STK11 activity to induce anti-HER2 therapy resistance in breast cancer
Resistance to HER2-targeted therapies presents a major challenge in the treatment of patients with HER2-positive breast cancer. N(6)-methyladenosine (m6A) modification plays a critical role in tumor progression; however, its role in mediating resistance to anti-HER2 therapy remains poorly defined. In trastuzumab-resistant HER2-positive breast cancer tissues, METTL14 expression is significantly upregulated and correlates with poor trastuzumab response. Moreover, our data suggest that the transcription factor RAD21 directly regulates METTL14 expression by binding to its promoter region. Elevated METTL14 expression enhances resistance to HER2-targeted therapies, while METTL14 knockdown restores trastuzumab sensitivity in resistant breast cancer cells. Mechanistically, METTL14 facilitates m6A methylation of STK11 mRNA, increasing its stability in an m6A-dependent manner, thereby contributing to resistance. Taken together, our findings define a novel RAD21-METTL14-STK11 axis that drives trastuzumab resistance in HER2-positive breast cancer and highlight potential therapeutic targets for overcoming treatment failure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


