自引入医学研究所标准:一项系统调查以来,治疗临床实践指南的可信度略有提高。
IF 5.2
2区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
摘要
目的:探讨自医学研究所(IOM)标准引入以来,治疗指南的可信度发生了多大程度的变化。研究设计和设置:在这项系统调查中,我们检索了Medline、Embase、PsycINFO和Trip,检索了2010年1月至12月(iom前)和2022年(iom后)的指南,这些指南是通过对证据的系统评价得出的,以英文撰写,包括对任何年龄个体的任何健康状况的治疗建议。使用10项修改后的国家指南信息中心可信赖标准遵守程度(NEATS)工具,成对审稿人独立评估了2010年和2022年随机选择的70项指南的可信赖性。我们计算了2010年和2022年指南中四个关键项目的平均得分、所有项目的平均得分和5点李克特量表中单个项目的得分的平均差异(MD)和相应的95%置信区间(CI),其中1为最低依从性,5为最高依从性。我们还探讨了在关键项目中达到平均4分或更高分数的指南比例的变化。结果:关键项目的平均得分从2.28增加到2.74 (MD 0.46, 95% CI 0.13至0.79),所有项目的平均得分从2.20增加到2.52 (MD 0.32, 95% CI 0.08至0.56)。在关键项目上平均得分为4分或更高的指南比例从2.9%增加到18.6%(绝对差异:15.7%,95% CI: 5.8%至25.6%)。平均得分增幅最大的项目包括资金来源披露和财务coi的披露和管理,其次是证据综合、研究选择和搜索策略的增幅较小。其他项目与2010年相比几乎没有变化。结论:通过改进的NEATS工具进行评估,尽管在引入IOM标准后治疗指南的可信度有所提高,但改进证明是适度的,并且后IOM指南的可信度仍然是次优的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
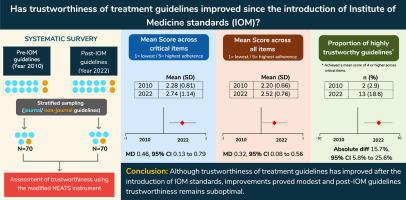
Trustworthiness of treatment clinical practice guidelines has modestly improved since the introduction of Institute of Medicine standards: a systematic survey
Objectives
To explore the extent to which trustworthiness of treatment guidelines has changed since the introduction of Institute of Medicine (IOM) standards.
Study Design and Setting
In this systematic survey, we searched Medline, Embase, PsycINFO, and Trip from January to December 2010 (pre-IOM) and 2022 (post-IOM) for guidelines that were informed by a systematic review of evidence, written in English, and included recommendations for the treatment of any health conditions in individuals of any age. Using the 10-item modified National Guideline Clearinghouse Extent of Adherence to Trustworthy Standards (NEATS) instrument, paired reviewers independently assessed trustworthiness of 70 randomly selected guidelines from 2010 and 70 from 2022. We calculated mean difference (MD) and corresponding 95% confidence interval (CI) comparing guidelines from 2010 with 2022 for mean score across four critical items, mean score across all items, and score of individual items on 5-point Likert scales in which 1 was least adherence and 5, highest adherence. We also explored change in the proportion of guidelines that achieved a mean score of 4 or higher across critical items.
Results
The average of mean scores increased from 2.28 to 2.74 (MD: 0.46, 95% CI: 0.13–0.79) across critical items and from 2.20 to 2.52 (MD: 0.32, 95% CI: 0.08–0.56) across all items. The proportion of guidelines with a mean score of 4 or higher across critical items increased from 2.9% to 18.6% (absolute difference: 15.7%, 95% CI: 5.8%–25.6%). The items with the greatest increase in the average score included disclosure of funding sources and disclosure and management of financial conflict of interests, followed by a smaller increase in synthesis of evidence, study selection, and search strategy. Other items showed little to no change from 2010.
Conclusion
As assessed by the modified NEATS instrument, although trustworthiness of treatment guidelines has improved after the introduction of IOM standards, improvements proved modest and post-IOM guidelines trustworthiness remains suboptimal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Clinical Epidemiology
医学-公共卫生、环境卫生与职业卫生
CiteScore
12.00
自引率
6.90%
发文量
320
审稿时长
44 days
期刊介绍:
The Journal of Clinical Epidemiology strives to enhance the quality of clinical and patient-oriented healthcare research by advancing and applying innovative methods in conducting, presenting, synthesizing, disseminating, and translating research results into optimal clinical practice. Special emphasis is placed on training new generations of scientists and clinical practice leaders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


