推进重组抗体在大肠杆菌中的生产:利用双GFP启动子和成像技术优化表达和纯化。
IF 1.2
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
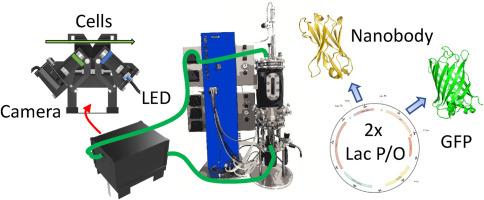
补料分批发酵结果高重组蛋白滴度在有限的培养体积。因此,它是生物加工行业的首选操作模式。在生物加工中,优化饲养、诱导和收获时间是一个非常耗时的挑战,因为表达的目标蛋白很少能实时检测到。在这项研究中,描述了一个在线传感器的构建,集成了双GFP启动子结构,一个蓝色LED和一个树莓派相机,用于实时监测重组抗体在大肠杆菌中的表达。双启动子结构允许在细胞质中同时表达GFP和在细胞质中同时表达重组抗体,从而可以使用GFP荧光作为蛋白质产量的代理。随着时间的推移,GFP荧光与Fab和纳米体的表达相关,荧光的相对数量预测了诱导程度。在纳米体分批补料发酵中,dGFP/dt的下降速率是确定最佳收获点、减少过多孵育时间和减少纳米体泄漏到培养基中的一个有价值的参数。进一步证明,用树莓派8 MP相机在流池中捕获的RGB图像的像素值的定量导致GFP检测的灵敏度与μPMT和光电二极管传感器相同。3d打印GFP传感器站是过程优化和通过实时可视化启动子激活教育生物过程工程专业学生的宝贵工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Advancing recombinant antibody production in E. coli: Optimization of expression and purification via dual GFP promoter and imaging technology
Fed-batch fermentation results in high recombinant protein titers in limited culture volumes. Therefore, it is the preferred operation mode in the bioprocess industry. Optimizing feeding, induction, and harvest timing is a significant time-consuming challenge in bioprocessing complicated by the fact that expressed target protein is rarely detectable in real-time. In this study, the construction of an online sensor is described integrating a dual GFP promoter construct, a blue LED and a Raspberry Pi camera for real-time monitoring of recombinant antibody expression in Escherichia coli. The dual promoter construct allows simultaneous expression of GFP in the cytoplasm and the recombinant antibody in the periplasm, enabling the use of GFP fluorescence as a proxy for protein yield. GFP fluorescence correlated with Fab and nanobody expression over time and the relative quantity of fluorescence predicted the extent of induction. In nanobody fed-batch fermentations, the decreasing rate of dGFP/dt was a valuable parameter for identifying the optimal harvest point, minimizing excessive incubation time and reducing nanobody leakage into the medium. It was further demonstrated that quantitation of pixel values from RGB images captured with a Raspberry Pi 8 MP camera in the flow cell resulted in equal sensitivity for GFP detection as that achieved with a μPMT and photodiode sensors. The 3D-printable GFP sensor station is a valuable tool for process optimization and for educating bioprocess engineering students through real-time visualization of promoter activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Protein expression and purification
生物-生化研究方法
CiteScore
3.70
自引率
6.20%
发文量
120
审稿时长
32 days
期刊介绍:
Protein Expression and Purification is an international journal providing a forum for the dissemination of new information on protein expression, extraction, purification, characterization, and/or applications using conventional biochemical and/or modern molecular biological approaches and methods, which are of broad interest to the field. The journal does not typically publish repetitive examples of protein expression and purification involving standard, well-established, methods. However, exceptions might include studies on important and/or difficult to express and/or purify proteins and/or studies that include extensive protein characterization, which provide new, previously unpublished information.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


