aav介导的MUC5AC siRNA递送预防哮喘患者粘膜纤毛功能障碍。
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
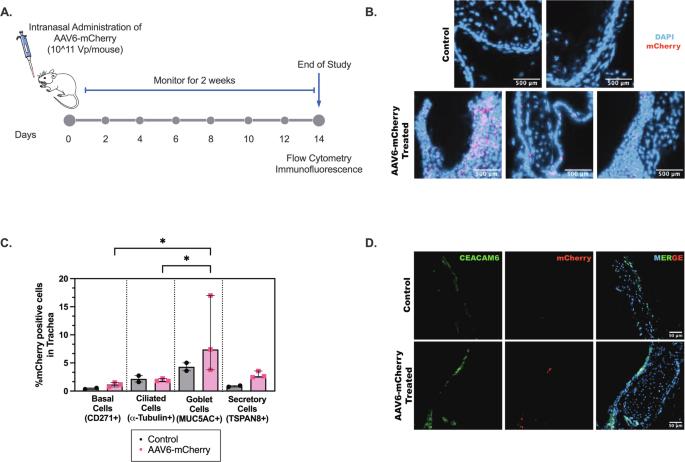
肺中产生的黏液的主要结构成分是黏液蛋白5B (MUC5B)和黏液蛋白5AC (MUC5AC),其中MUC5B在健康人群中表达相对较高。在哮喘患者的肺中,MUC5B向MUC5AC转变,MUC5AC是主要分泌的粘蛋白,已被证明会损害粘睫清除(MCC)并增加气道粘液塞的形成。考虑到MUC5AC在哮喘性肺部疾病中的作用,MUC5AC代表了一个潜在的治疗靶点,可以利用基因传递方法来调节其表达。为此,我们探索了腺相关病毒血清型6 (AAV6)作为一种病毒基因载体,通过siRNA传递转导气道上皮细胞并降低MUC5AC的表达。我们证实了AAV6能够在体外转导上皮细胞以及健康小鼠的气道,并在分泌粘液的杯状细胞中有高的转基因表达。通过多颗粒跟踪分析,我们观察到AAV6能够穿透正常和muc5ac富集的粘液屏障。携带muc5ac靶向siRNA的AAV6在IL-13攻击前作为HAE细胞培养的预防性治疗进行了评估。与未处理的对照组相比,用AAV6-MUC5AC siRNA处理的IL-13刺激的HAE培养显著降低了MUC5AC mRNA和蛋白的表达。在AAV6-MUC5AC siRNA治疗后,IL-13刺激的HAE培养物中的粘膜纤毛运输也保持不变,与健康对照组相当。总之,这些发现支持AAV6可以作为一种吸入基因疗法来抑制MUC5AC的过表达,并恢复哮喘患者正常的气道清除功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
AAV-mediated MUC5AC siRNA delivery to prevent mucociliary dysfunction in asthma
The main structural components of mucus produced in the lung are mucin 5B (MUC5B) and mucin 5AC (MUC5AC) where a relatively higher expression of MUC5B is typical in health. In the lungs of individuals with asthma, there is a shift from MUC5B to MUC5AC as the predominantly secreted mucin which has been shown to impair mucociliary clearance (MCC) and increase airway mucus plug formation. Given its role in asthmatic lung disease, MUC5AC represents a potential therapeutic target where a gene delivery approach could be leveraged to modulate its expression. For these purposes, we explored adeno-associated virus serotype 6 (AAV6), as a viral gene vector to transduce airway epithelial cells and reduce MUC5AC expression via siRNA delivery. We confirmed that AAV6 was able to transduce epithelial cells in vitro as well as in the airways of healthy mice in vivo with high transgene expression in mucus-secreting goblet cells. Using multiple particle tracking analysis, we observed that AAV6 was capable of penetrating both normal and MUC5AC-enriched mucus barriers. AAV6 carrying MUC5AC-targeting siRNA was evaluated as a prophylactic treatment in HAE cell cultures before IL-13 challenge. IL-13 stimulated HAE cultures treated with AAV6-MUC5AC siRNA had significantly reduced MUC5AC mRNA and protein expression compared to untreated controls. Mucociliary transport in IL-13 stimulated HAE cultures was also maintained and comparable to healthy controls following AAV6-MUC5AC siRNA treatment. Together, these findings support that AAV6 may be used as an inhaled gene therapy to suppress MUC5AC overexpression and restore normal airway clearance function in asthma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


