MicroRNA-15b通过靶向NFS1调控线粒体自噬促进肌筋膜疼痛综合征。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景和目的:肌筋膜疼痛综合征(MPS)是由肌筋膜触发点(MTrPs)功能障碍引起的,其机制尚不清楚。本研究旨在探讨mir - 15b在MTrP发病机制中的功能,重点关注其对铁硫(Fe-S)簇合成和有丝分裂的调控。方法:采用重复机械损伤和偏心运动建立大鼠MTrP模型。分析骨骼肌组织和原代卫星细胞的miR-15b表达、Fe-S簇相关蛋白(NFS1、NDUFS3和SDH B)和线粒体自噬标志物(FUN14结构域蛋白1 (FUNDC1)和LC3-II/I)。在体外,使用肿瘤坏死因子-α (TNF-α)诱导的炎症和miR-15b调节(模拟/海绵)和NFS1调节(过表达/敲低)来评估线粒体功能。在体内,通过肌肉注射瞬时转染的NFS1质粒、miR-15b质粒或海绵构建物的复合物来评估正常和MTrP模型大鼠的治疗效果。结果:MTrP大鼠表现出miR-15b,抑制NFS1, fe - s依赖性复合物受损。双荧光素酶检测证实了miR-15b靶向NFS1。救援实验进一步验证了miR-15b直接抑制NFS1,增加活性氧(ROS),降低线粒体膜电位(MMP),触发fundc1介导的线粒体自噬。TNF-α刺激升高miR-15b水平,加剧线粒体功能障碍,而miR-15b抑制恢复NFS1并使线粒体自噬正常化。在正常大鼠中,miR-15b过表达再现了健康大鼠的mtrp样病理。此外,在MTrP模型大鼠中,miR-15b过表达加重了这些表现,海绵和NFS1治疗减轻甚至逆转了某些病理改变。结论:miR-15b通过抑制NFS1、破坏Fe-S稳态和激活依赖于fundc1的线粒体自噬来驱动MTrP进展。靶向miR-15b可减轻线粒体功能障碍和疼痛超敏反应,强调其在MPS中的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
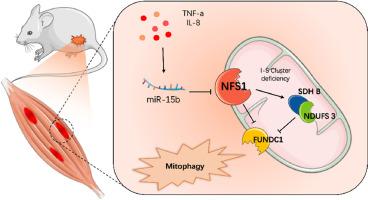
MicroRNA-15b promotes myofascial pain syndrome by targeting NFS1 to regulate mitophagy
Background and aims
Myofascial pain syndrome (MPS), driven by dysfunction in myofascial trigger points (MTrPs), remains mechanistically unclear. This study aimed to explore miR-15 b's function in MTrP pathogenesis, focusing on its regulation of iron-sulfur (Fe–S) cluster synthesis and mitophagy.
Methods
A rat MTrP model was established using repetitive mechanical injury and eccentric exercise. Skeletal muscle tissues and primary satellite cells were analysed for miR-15b expression, Fe-S cluster-related proteins (NFS1, NDUFS3, and SDH B), and mitophagy markers (FUN14 structural domain-containing protein 1 (FUNDC1) and LC3-II/I). In vitro, tumour necrosis factor-alpha (TNF-α)-induced inflammation and miR-15b modulation (mimics/sponges) and NFS1 modulation (overexpression/knockdown) were used to assess mitochondrial functions. In vivo, the therapeutic effect on normal and MTrP model rats was evaluated by intramuscular injection of transiently transfected complexes of NFS1 plasmid, miR-15b plasmid or sponge constructs.
Results
MTrP rats exhibited miR-15b, suppressed NFS1, and impaired Fe-S-dependent complexes. Dual luciferase assays verified miR-15b targeting NFS1. Rescue experiments further validated that miR-15b directly inhibits NFS1, increase reactive oxygen species (ROS), lowering mitochondrial membrane potential (MMP), triggering FUNDC1-mediated mitophagy. TNF-α stimulation elevated miR-15b levels, exacerbating mitochondrial dysfunction, whereas miR-15b inhibition restored NFS1 and normalised mitophagy. In normal rats, miR-15b overexpression recapitulated MTrP-like pathology in healthy rats. Moreover, in the MTrP model rats, miR-15b overexpression exacerbated these manifestations, sponge and NFS1 treatment attenuated or even reversed certain pathological changes.
Conclusions
miR-15b drives MTrP progression by suppressing NFS1, disrupting Fe-S homeostasis, and activating FUNDC1-dependent mitophagy. Targeting miR-15b mitigates mitochondrial dysfunction and pain hypersensitivity, underscoring its therapeutic potential in MPS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


