早发性结直肠癌与晚发性结直肠癌肿瘤的不同代谢和遗传改变
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
早发性结直肠癌(EO-CRC)发生在50岁以下的个体在全球范围内迅速增加,而晚发性结直肠癌(LO-CRC)的发病率近年来有所下降。先前的研究已经确定了与CRC生物学相关的代谢物,但是EO-CRC和LO-CRC之间的肿瘤特异性差异尚未被探索。本研究旨在比较EO-CRC和LO-CRC患者的肿瘤代谢组,揭示EO-CRC独特的生化状态。对EO-CRC (n=53)和LO-CRC (n=314)患者的肿瘤和患者匹配的正常组织进行基于质谱的非靶向代谢组学研究,以鉴定肿瘤中显著改变的代谢物(q≤0.05)。我们进行了代谢物集富集分析、代谢途径和网络分析,以确定改变的代谢物与生物学功能之间的关系。分析显示,156种代谢物在正常组织和肿瘤组织之间发生了显著变化。多巴胺的代谢物同质香草酸(HVA)在EO-CRC中被下调。尽管在EO- crc和LO-CRC之间有共同的hva代谢基因变化,但儿茶酚胺代谢的破坏可能是EO- crc生物学特有的。基因表达验证支持的通路和网络分析显示,PD-L1在EO-CRC中唯一降低,提示免疫抑制。此外,磷脂信号在EO-CRC中得到支持,而LO-CRC肿瘤显示EGFR信号和氧化应激相关基因的改变。综上所述,本研究揭示了EO-CRC和LO-CRC患者肿瘤组织中代谢的细微差别,提示EO-CRC的生物学中儿茶酚胺代谢、磷脂信号传导和免疫抑制。这些发现为eo -CRC的代谢提供了新的见解,可能为这组CRC患者提供新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
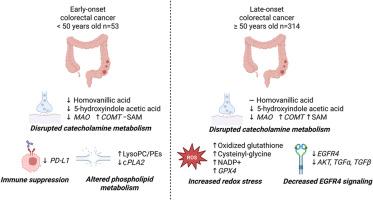
Distinct metabolic and genetic alterations in tumors from early-onset versus late-onset colorectal cancer
Early-onset colorectal cancer (EO-CRC) occurring in individuals under age 50 is rapidly increasing globally, while the incidence of late-onset colorectal cancer (LO-CRC) has decreased over recent years. Previous studies have identified metabolites linked to CRC biology, however tumor-specific differences between EO-CRC and LO-CRC have not been explored. This study aimed to compare the tumor metabolome of EO-CRC and LO-CRC patients to reveal the unique biochemical state of EO-CRC. Mass spectrometry-based untargeted metabolomics was performed on tumor and patient-matched normal tissues from EO-CRC (n = 53) and LO-CRC (n = 314) patients to identify metabolites significantly altered in tumors (q ≤ 0.05). Metabolite set enrichment analysis, metabolic pathway, and network analyses were performed, to identify the relationship between the altered metabolites and biological function. Analysis revealed 155 metabolites significantly altered between normal and tumor tissues. Homovanillic acid , a metabolite of dopamine, was uniquely downregulated in EO-CRC. Despite shared changes to homovanillic acid-metabolizing genes between EO- and LO-CRC the disruption in catecholamine metabolism may be specific to EO-CRC biology. Pathway and network analysis, supported by gene expression validation, showed that PD-L1 was uniquely decreased in EO-CRC suggesting immunosuppression. Additionally, phospholipid signaling was favored in EO-CRC, whereas LO-CRC tumors showed alterations to EGFR signaling and oxidative stress-related genes. In summary, this study reveals the metabolic nuances in tumor tissues from patients with EO-CRC and LO-CRC, indicating catecholamine metabolism, phospholipid signaling and immunosuppression in the biology of EO-CRC. These findings provide new insight into the metabolism of EO-CRCs that may inform new therapeutic strategies for this group of CRC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


