靶向KEAP1/NRF2与橄榄苦苷的相互作用通过抑制巨噬细胞铁凋亡来改善动脉粥样硬化。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
动脉粥样硬化(AS)是一种慢性炎症性动脉疾病。橄榄苦苷从橄榄叶中提取,具有广泛的心脏保护作用。然而,OL对AS的影响仍有待充分阐明。用ox-LDL培养巨噬细胞体外模拟斑块内巨噬细胞损伤。将编码短发夹RNA靶向NRF2 (AAV9- shnrf2)和AAV-KEAP1-R451S的重组腺相关病毒血清型9 (AAV9)给予ol处理的ApoE-/-小鼠。通过免疫沉淀、Chip-qPCR、海马实验和蛋白质组学分析探索其分子机制。OL可以显著减缓AS的进展,增强斑块的稳定性,这可以通过斑块面积和脂质沉积的减少以及胶原含量的增加来证明。蛋白质组学显示,OL通过上调GPX4/xCT水平,改善线粒体功能,减轻氧化应激,抑制ox-LDL和动脉粥样硬化斑块处理的巨噬细胞脂质过氧化,从而抑制铁凋亡。此外,OL增强了NRF2的激活和核易位,而NRF2的抑制或敲低则消除了OL对AS和巨噬细胞铁凋亡的保护作用。机制上,分子动力学分析表明OL可能竞争性地结合KEAP1的Arg415位点,促进NRF2与KEAP1的分离和核易位。此外,转染Arg415突变质粒(KEAP1-R415S)可消除ox-LDL诱导的巨噬细胞中OL的抗氧化和抗铁凋亡作用。此外,在AAV-KEAP1-R415S突变型ApoE-/-小鼠中,OL对动脉粥样硬化和巨噬细胞铁凋亡的保护作用显著减弱。OL通过竞争性地结合KEAP1的Arg415残基,促进NRF2核易位和活化,从而减轻AS进展和巨噬细胞铁凋亡。这些发现为治疗AS的潜在治疗策略提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
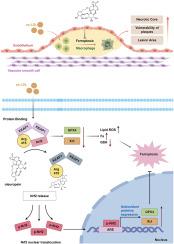
Targeting KEAP1/NRF2 interaction with oleuropein ameliorates atherosclerosis by inhibiting macrophage ferroptosis
Atherosclerosis (AS) is a chronic inflammatory arterial disease. Oleuropein (OL), extracted from olive leaves, has demonstrated broad cardioprotective properties. However, the effects of OL on AS remain to be fully elucidated. ox-LDL incubated macrophages were used to imitate macrophage damage within plaques in vitro. Recombinant adeno-associated virus serotype 9 (AAV9) encoding a short hairpin RNA targeting NRF2 (AAV9-shNRF2) and AAV-KEAP1-R451S were administered to OL-treated ApoE−/− mice. The molecular mechanisms were explored through immunoprecipitation and Chip-qPCR, seahorse assays, and proteomics analysis. OL administration significantly attenuated the progression of AS and enhanced plaque stability, as evidenced by reduced plaque area and lipid deposition, along with increased collagen content. Proteomics revealed that OL suppressed ferroptosis by upregulating GPX4/xCT level, improving mitochondrial function, alleviating oxidative stress and suppressing lipid peroxidation in macrophages treated by ox-LDL and atherosclerotic plaques. Moreover, OL enhanced NRF2 activation and nuclear translocation, while NRF2 inhibition or knockdown abolished the protective effect of OL on AS and macrophage ferroptosis. Mechanistically, molecular dynamics analysis suggested that OL may bind to the Arg415 site of KEAP1 competitively, promoting the separation and nuclear translocation of NRF2 from KEAP1. Additionally, transfection with the Arg415 mutant plasmid (KEAP1-R415S) abolished the antioxidative and anti-ferroptosis effects of OL in macrophages induced by ox-LDL. Moreover, the protective effects of OL against atherosclerosis and macrophage ferroptosis were significantly attenuated in AAV-KEAP1-R415S mutant ApoE−/− mice. OL attenuated AS progression and macrophage ferroptosis by facilitating NRF2 nuclear translocation and activation via binding to the Arg415 residue of KEAP1 competitively. These findings identified a novel insight into potential therapeutic strategies for the treatment of AS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


