Sestrin2通过调节线粒体动力学减轻败血症诱导的树突状细胞免疫抑制。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:线粒体动力学和线粒体自噬是炎症疾病中维持线粒体质量和稳态的关键机制,尽管它们在炎症调节中的激活途径尚不清楚。Sestrin2 (Sesn2)是一种对细胞稳态至关重要的应激反应蛋白,本研究调查了其在脓毒症期间线粒体动力学中的调节作用及其在树突状细胞(DC)坏死性坏死中的潜在机制。方法:本研究利用Western blotting、激光共聚焦显微镜和透射电镜对脓毒症DCs中sesn2调控的线粒体动力学蛋白如动力蛋白相关蛋白1 (DRP1)、线粒体裂变因子(MFF)和丝裂蛋白2 (MFN2)进行了评价。我们建立了慢病毒转染细胞系和Sesn2敲除小鼠模型,以评估Sesn2缺失在DC坏死性坏死中的作用及其对脓毒症攻击后免疫反应信号通路的影响。结果:盲肠结扎和穿孔(CLP)引起的脓毒症和脂多糖(LPS)刺激均引起线粒体动力学的显著改变,Sesn2表达在24小时达到峰值。当Sesn2基因被敲除时,DCs的坏死下垂和线粒体分裂明显增加,而线粒体融合减少。相反,Sesn2过表达对DCs具有显著的保护作用。与野生型(WT)小鼠相比,Sesn2基因缺陷小鼠的坏死下垂、DCs免疫抑制和7天死亡率均显著增加。此外,sesn2介导的dc上线粒体融合分裂与坏死性凋亡通路密切相关,DRP1-ROS-ZBP1信号明显参与脓毒症下dc坏死性凋亡的下调。结论:脓毒症背景下,sesn2介导的线粒体融合分裂可通过DRP1-ROS-ZBP1通路显著激活,减轻DCs的坏死性下垂。因此,Sesn2稳定的线粒体动力学可能有助于逆转与脓毒症并发症相关的免疫抑制,这一点很重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
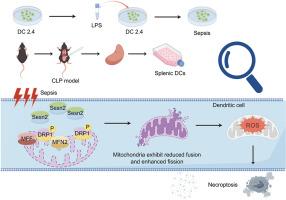
Sestrin2 alleviates sepsis-induced immunosuppression of dendritic cells by regulating mitochondrial dynamics
Background
Mitochondrial dynamics and mitophagy are key mechanisms maintaining mitochondrial quality and homeostasis in inflammatory diseases, though their activation pathways in inflammatory regulation remain unclear. Sestrin2 (Sesn2), a stress-responsive protein critical for cellular homeostasis, was investigated in this study for its regulatory role in mitochondrial dynamics during sepsis and its potential mechanism in dendritic cell (DC) necroptosis.
Methods
This study evaluated Sesn2-regulated mitochondrial dynamics proteins such as dynamin-related protein 1 (DRP1), mitochondrial fission factor (MFF), and mitofusin 2 (MFN2) in DCs during sepsis using Western blotting, laser confocal microscopy, and transmission electron microscopy. Lentiviral-transfected cell lines and Sesn2-knockout mouse models were developed to assess Sesn2 deletion's role in DC necroptosis and its impact on immune response signaling pathways post-septic challenge.
Results
Both cecal ligation and perforation (CLP)-induced sepsis and lipopolysaccharide (LPS) stimulation elicited significant alterations in mitochondrial dynamics, and Sesn2 expression peaked at 24 h. When Sesn2 was knocked down, necroptosis and mitochondrial fission of DCs were noticeably increased, while mitochondrial fusion was decreased. Conversely, the overexpression of Sesn2 exerted a significant protective impact on DCs. Consistently, the necroptosis and immunosuppression of DCs and 7-days mortality rate in Sesn2 gene-deficient mice were significantly increased compared with those in wild-type (WT) mice. Furthermore, Sesn2-mediated mitochondrial fusion and division on DCs was identified to be closely associated with the necroptosis pathway, and DRP1-ROS-ZBP1 signaling was obviously involved in down-regulating necroptosis of DCs in the setting of sepsis.
Conclusions
Sesn2-mediated mitochondrial fusion and division can be significantly activated to alleviate the necroptosis of DCs via the DRP1-ROS-ZBP1 pathway in the context of sepsis. Thus, it is of importance that Sesn2 stabilized mitochondrial dynamics might be beneficial for reversing immunosuppression associated with septic complications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


