高度简化的微生物群对产后肠道、免疫和大脑发育的影响。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
在出生时,新生哺乳动物进入了一个高度微生物化的世界,导致数万亿微生物在肠道中定植,被称为微生物群。我们之前报道过无菌小鼠在出生后的头几天就改变了大脑发育,这表明出生时微生物的到来对正常的大脑发育至关重要。然而,GF小鼠是一种高度人工的模型系统,在免疫和肠道发育方面存在已知缺陷。为了确定更现实的减少微生物暴露的影响,我们使用了减少的,定义的微生物群。具体来说,GF小鼠坝被改变的Schaedler菌群(ASF)定植,这是一个由8种细菌组成的群落,在成年期支持相对正常的免疫和肠道功能。然后在ASF、GF和常规定植(即对照)小鼠出生的后代(P)3或P23天)中比较外周(结肠组织学和基因表达、血浆细胞因子)和脑(神经元细胞死亡、小胶质细胞密度、细胞因子和血脑屏障蛋白基因的前脑表达)的测量,重点关注先前报道的CC和GF条件之间的差异。我们观察到ASF新生儿在结肠中的细菌载量高于CC小鼠,但基本上是单菌落。对于某些指标(如血浆细胞因子、神经元细胞死亡),P3期的ASF新生儿与对照组相似,而对于其他新生儿则是gf样或中等水平。与CC后代的爆炸性多样化相比,ASF微生物群在断奶时增长到包括5种细菌。P23的分析表明,ASF断奶仔猪的小胶质细胞密度与对照组相似,但一些外周测量仍与gf相似。因此,缺乏微生物群的影响在生命的几天内是明显的,并且高度简化,定义的微生物群定殖使发育中的外周和大脑中的一些(但不是全部)测量正常化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
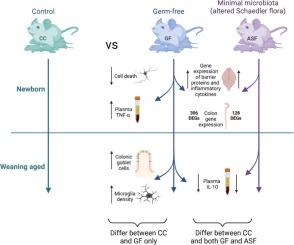
Effects of a highly simplified microbiota on postnatal intestinal, immune, and brain development
At birth, the newborn mammal enters a highly microbial world, leading to colonization of the gut by trillions of microorganisms, known as the microbiota. We previously reported that germ-free (GF) mice have altered brain development in the first days of life, suggesting that the arrival of microbes at birth is essential for normal brain development. However, GF mice are a highly artificial model system, with known deficits in immune and intestinal development. To determine the effects of a more realistic curtailment of microbial exposure, we utilized a reduced, defined microbiota. Specifically, GF mouse dams were colonized with the altered Schaedler flora (ASF), a community of eight bacterial species, which supports relatively normal immune and intestinal function in adulthood. Measures in the periphery (colon histology and gene expression, plasma cytokines) and brain (neuronal cell death, microglial density, forebrain expression of cytokine and blood–brain barrier protein genes) were then compared among offspring born to ASF, GF, and conventionally colonized (CC; i.e., control) mice at postnatal day (P)3 or P23, focusing on those measures previously reported to differ between CC and GF conditions. We observed that ASF newborns have a higher bacterial load in the colon than CC mice but are essentially monocolonized. For some measures (e.g., plasma cytokines, neuronal cell death), ASF newborns at P3 are similar to controls, whereas for others they are GF-like or intermediate. The ASF microbiota grows to comprise 5 bacterial species by weaning age, compared to explosive diversification in CC offspring. Analyses at P23 demonstrate that microglia density is like that of controls in ASF weanlings but several peripheral measures remain GF-like. Thus, effects of an absent microbiota are evident within a few days of life, and colonization with a highly simplified, defined microbiota normalizes some, but not all, measures in the developing periphery and brain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


