酮单酯通过先天免疫IFITM3途径减轻阿尔茨海默病转基因小鼠模型的认知障碍
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
阿尔茨海默病(AD)是一种以不可逆的认知能力下降和脑功能障碍为特征的进行性神经退行性疾病,由于其难以捉摸的发病机制和缺乏改善疾病的治疗方法,仍然是一个重大的全球健康挑战。越来越多的证据强调酮体,特别是β-羟基丁酸(β-HB)的神经保护潜力,因为它们在减轻ad相关病理方面具有多种生物学作用。最近的研究发现,干扰素诱导的跨膜蛋白3 (IFITM3)是淀粉样蛋白-β (a β)形成的关键调节因子,也与先天免疫有关。本研究通过给药BD-AcAc2诱导APP/PS1转基因小鼠外源性酮症,并利用a β刺激的星形胶质细胞探讨β-HB作为营养干预的机制作用。我们的研究结果表明,β-HB可能通过调节IFITM3通路,显著提高APP/PS1小鼠的认知能力,减少海马淀粉样斑块负担。此外,β-HB可减弱a β诱导的IFITM3激活,降低活性氧(ROS)水平,下调星形胶质细胞中促炎细胞因子的表达。总的来说,这些结果证实了β-HB是一种通过ifitm3介导的机制起作用的神经保护剂,为针对AD中代谢-免疫相互作用的治疗策略提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
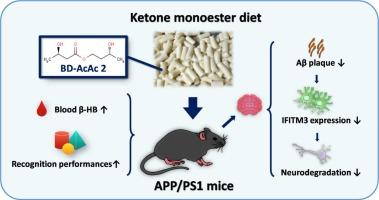
Ketone monoester alleviates cognitive impairment in a transgenic mouse model of Alzheimer’s disease through innate immunological IFITM3 pathway
Alzheimer’s disease (AD), a progressive neurodegenerative disorder characterized by irreversible cognitive decline and cerebral dysfunction, remains a major global health challenge due to elusive pathogenesis and the lack of disease-modifying therapies. Growing evidence underscores the neuroprotective potential of ketone bodies, particularly β-hydroxybutyrate (β-HB), owing to their diverse biological roles in mitigating AD-related pathology. Recent advances also implicate innate immunity in AD progression, identifying interferon-induced transmembrane protein 3 (IFITM3) as a pivotal regulator of amyloid-β (Aβ) formation. In this study, BD-AcAc2 was administered to induce exogenous ketosis in APP/PS1 transgenic mice, and Aβ-stimulated astrocytes were employed to explore the mechanistic role of β-HB as a nutritional intervention. Our findings demonstrate that β-HB significantly enhanced cognitive performance and reduced hippocampal amyloid plaque burden in APP/PS1 mice, likely through modulation of the IFITM3 pathway. Furthermore, β-HB attenuated Aβ-induced IFITM3 activation, decreased reactive oxygen species (ROS) levels, and downregulated pro-inflammatory cytokine expression in astrocytes. Collectively, these results establish β-HB as a neuroprotective agent that acts through IFITM3-mediated mechanisms, providing novel insights into therapeutic strategies targeting metabolic-immune interactions in AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


