31P固体核磁共振研究仿生基质囊泡中TNAP活性和磷灰石形成的调节。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
骨骼和牙齿矿化依赖于一个精确调控的事件序列,最终导致磷灰石沉积到胶原原纤维上。基质囊泡(Matrix vesicles, MVs)是矿化能力细胞释放的细胞外囊泡,通过碱性磷酸酶(TNAP)的催化活性在这一过程中发挥关键作用。mv的脂质组成,特别是磷脂酰丝氨酸(PS)-钙复合物,促进了无定形磷酸钙的成核和磷灰石的形成。然而,TNAP结构、脂膜环境及其酶活性之间的相互作用仍不完全清楚。利用双棕榈酰磷脂酰胆碱(DPPC)和各种TNAP突变体构建的MVs蛋白脂质体的仿生模型,研究了TNAP的活性和矿化潜力。分子对接和定点诱变表明,在TNAP的催化位点和锚定位点附近的特定半胱氨酸取代会影响结构稳定性、酶活性和脂质双分子层的掺入。值得注意的是,TNAP突变体S221C和P307C在DPPC脂质体中表现出增强的催化效率,而A420C由于催化位点附近的位阻而表现出活性降低。固体核磁共振和低温透射电镜分析证实了羟基磷灰石的形成,脂质锚定的TNAP对矿化过程有重要贡献。这些发现强调了脂质环境对TNAP功能特性的关键影响,并提供了对生物矿化和相关病理(包括与各种TNAP突变相关的低磷酸症)的机制的见解。该研究强调了ATP和TNAP水解焦磷酸盐在调节磷灰石形成中的重要性,并揭示了特定TNAP突变在调节酶活性、稳定性和矿物繁殖中的作用。了解这些相互作用可能会导致治疗和再生医学的替代治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
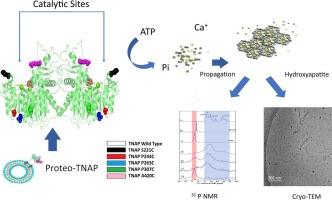
Modulation of TNAP activity and apatite formation in biomimetic matrix vesicles studied by 31P solid-state NMR
Skeletal and dental mineralization relies on a precisely regulated sequence of events culminating in apatite deposition onto collagen fibrils. Matrix vesicles (MVs), extracellular vesicles released by mineralization-competent cells, play a pivotal role in this process through the catalytic activity of alkaline phosphatase (TNAP). The lipid composition of MVs, particularly phosphatidylserine (PS)-calcium complexes, facilitates the nucleation of amorphous calcium phosphate and apatite formation. However, the interplay between the TNAP structure, the lipid membrane environment, and its enzymatic activity remains incompletely understood.
Biomimetic models of MVs, as proteoliposomes made with dipalmitoylphosphatidylcholine (DPPC) and various TNAP mutants, were used to investigate the TNAP's activity and mineralization potential. Molecular docking and site-directed mutagenesis revealed that specific cysteine substitutions near TNAP's catalytic and anchoring sites influence structural stability, enzymatic activity, and incorporation into lipid bilayers. Notably, TNAP mutants S221C and P307C exhibited enhanced catalytic efficiency in DPPC liposomes, while A420C showed reduced activity due to steric hindrance near the catalytic site. Solid-state NMR and cryo-TEM analyses confirmed hydroxyapatite formation, with significant contributions from lipid-anchored TNAP to the mineralization process.
These findings highlight the critical influence of the lipid environment on TNAP's functional properties and provide insights into the mechanisms governing biomineralization and related pathologies, including hypophosphatasia associated with various TNAP mutations. The study underscores the importance of ATP and pyrophosphate hydrolysis by TNAP in modulating apatite formation and reveals the role of specific TNAP mutations in regulating enzymatic activity, stability, and mineral propagation. Understanding these interactions could lead to alternate therapeutic strategies in treatment and regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


