vi型胶原衍生宿主防御肽膜相互作用的生物物理研究。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
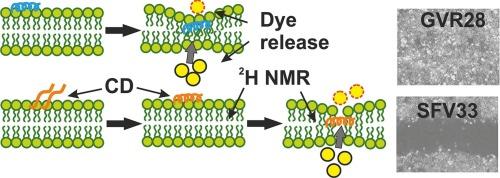
胶原VI是一种细胞外基质蛋白,在结缔组织中形成复杂的微纤维网络。具体来说,我们关注的是它在先天免疫中的作用,特别是α3(VI)链上的阳离子序列基序,它在体外和体内对革兰氏阳性和革兰氏阴性细菌都表现出很强的抗菌特性。细胞毒性试验显示,即使在对细菌病原体有效的浓度下,也几乎没有副作用。这种良好的安全性表明,这些抗菌肽选择性地靶向细菌膜,同时保留宿主细胞,使其成为治疗开发的有希望的候选者。利用固体核磁共振、CD和荧光光谱对两种抗菌肽的膜结构和相互作用进行了定量详细研究。与POPC/30 %胆固醇相比,POPE/POPG 3/1囊泡释放的钙黄蛋白更为明显,而当研究POPC/POPG 3/1脂质体时,这种活性增加了大约两个数量级。这种明显的脂质依赖性在magainin 2(一种众所周知的线性阳离子AMP)中得到了再现。在脂质滴定实验中,两种胶原来源的肽都表现出从主要随机线圈到螺旋构象的转变。膜关联的定量评估需要peg -脂质的存在,已知peg -脂质可以防止POPE/POPG 3/1脂质体的凝集。GVR28的解离常数在260 μM范围内,而结合等温线显示SFV33与细菌膜结合时处于中间状态。2H固态核磁共振显示,在POPE/POPG膜中氘化PG棕榈酰链存在明显的膜无序性。综合生物物理数据表明胶原衍生肽有两种不同的作用模式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biophysical investigations of the membrane interactions of collagen VI-derived host defense peptides
Collagen VI is an extracellular matrix protein forming complex microfibrillar networks in connective tissues. Specifically, we focused on its role in innate immunity, in particular on cationic sequence motifs from the α3(VI)-chain, which exhibit strong antibacterial properties against both Gram-positive and Gram-negative bacteria in vitro and in vivo. Cytotoxicity assays revealed minimal to no adverse effects, even at concentrations effective against bacterial pathogens. This favorable safety profile suggests that these antimicrobial peptides selectively target bacterial membranes while sparing host cells, making them promising candidates for therapeutic development.
The membrane structure and interactions of two antimicrobial peptides were investigated in quantitative detail using solid-state NMR, CD and fluorescence spectroscopies. Whereas calcein release was somewhat more pronounced from POPE/POPG 3/1 vesicles when compared to POPC/30 % cholesterol, this activity is about two orders of magnitude increased when POPC/POPG 3/1 liposomes are investigated. This pronounced lipid dependence was reproduced with magainin 2, a well-known linear cationic AMP. In lipid titration experiments both collagen-derived peptides showed a transition from predominantly random coil to helical conformations. Quantitative evaluation of membrane association required the presence of PEG-lipids which are known to prevent the agglutination of POPE/POPG 3/1 liposomes. A dissociation constant in the 260 μM range was observed for GVR28 while the binding isotherms reveal an intermediate state when SFV33 associates with bacterial membranes. 2H solid-state NMR reveals considerable membrane disorder of the deuterated PG palmitoyl chain in POPE/POPG membranes. The ensemble of biophysical data suggests two distinct modes of action for the collagen derived peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


