新型含氮杂环衍生物潜在抗癌药物的合成及生物学评价。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
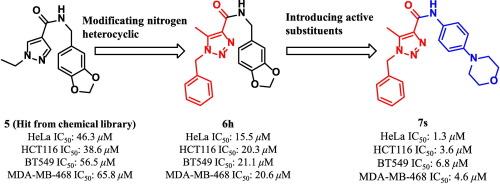
在筛选结果的基础上,设计合成了一系列含氮五元杂环化合物,用于抗癌评价。其中,7个 s具有1,2,3-三唑支架,对四种人类癌细胞系(IC₅0)表现出最有效的抗增殖活性本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of novel nitrogen-containing heterocyclic derivatives as potential anticancer agents
Based on screening results, a series of nitrogen-containing five-membered heterocyclic compounds were designed and synthesized for anticancer evaluation. Among them, 7s, featuring a 1,2,3-triazole scaffold, exhibited the most potent antiproliferative activity against four human cancer cell lines (IC₅₀ < 10 μM). Mechanistic studies revealed that 7s downregulated phosphorylated AKT (p-AKT) protein at 5 μM. It also demonstrated moderate metabolic stability (T₁/₂ = 38.9 min, clearance = 23.4 mL/min/kg) and acceptable aqueous solubility (2.6 mg/100 mL). SwissADME predictions confirmed its good oral bioavailability and gastrointestinal absorption. Importantly, 7s showed low cytotoxicity toward normal HEK293 cells (IC₅₀ = 56.2 μM), suggesting a favorable safety. These results support 7s as a promising lead compound for further development in anticancer drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


