真菌乙酰辅酶a合成酶抑制剂酰基amp磷酸同异构体的合成及评价。
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
乙酰辅酶a合成酶(ACS)是腺苷酸形成酶超家族的成员。这种酶在细胞代谢中起关键作用。虽然ACS酶在哺乳动物中不是必需的,但它们在一些真菌和寄生虫中是必需的,这些真菌和寄生虫对人类具有致病性。因此,抑制ACS酶是开发新型抗感染药物的一个新兴靶点。烷基AMP酯和酰基氨基酰基腺苷(酰基- ams)通过模拟酰基-AMP酶中间体是真菌ACS酶的有效抑制剂。进行分子对接研究以促进类似物的设计,并探索它们与ACS酶的潜在配体结合相互作用。合成了一系列酰基amp同异构体,并对其抑菌活性进行了筛选。值得注意的是,化合物14成功地与新型隐球菌ACS1酶结晶,为未来抑制剂的设计提供了有价值的结构见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
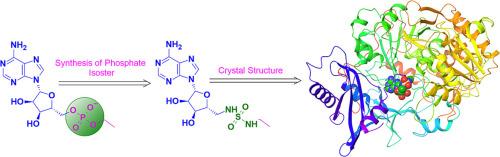
Synthesis and evaluation of acyl-AMP phosphate isosteres as inhibitors of fungal acetyl CoA synthetase
Acetyl-CoA synthetase (ACS) is a member of the adenylate-forming enzymes superfamily. This enzyme plays a crucial role in cellular metabolism. While ACS enzymes are non-essential in mammals, they are essential in some fungal species and parasites that are pathogenic to humans. Hence, inhibition of the ACS enzyme is an emerging target for the development of novel anti-infectives. Alkyl AMP esters and acyl sulfamoyl adenosine (Acyl-AMS) are potent inhibitors of fungal ACS enzymes by mimicingthe acyl-AMP enzyme intermediate. Molecular docking studies were performed to facilitate the design of analogs and to explore their potential ligand-binding interactions with the ACS enzyme. A series of acyl-AMP isosteres were synthesized and screened for inhibitory activity against fungal ACS enzymes. Notably, Compound 14 was successfully crystallized with the Cryptococcus neoformans ACS1 enzyme, providing valuable structural insight for future inhibitor design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


