SLC25A40通过增强nadph介导的脂质合成和抑制ROS积累诱导的铁凋亡来促进NSCLC的生长
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
线粒体是调节细胞代谢和维持氧化还原稳态的重要细胞器。它们的功能障碍与多种人类恶性肿瘤的进展密切相关。SLC25A40已被预测为谷胱甘肽进入线粒体所需的线粒体载体。然而,SLC25A40在人类癌症,特别是非小细胞肺癌(NSCLC)中的作用仍然知之甚少。我们发现SLC25A40在NSCLC中表达升高。这种上调与预后不良有关。沉默SLC25A40通过抑制细胞增殖和诱导铁下垂抑制NSCLC的生长,而过表达SLC25A40则促进NSCLC的生长。在机制上,SLC25A40通过增加nadph介导的脂质合成来促进细胞增殖,并通过减少线粒体ROS积累来抑制铁下垂。此外,我们证明SLC25A40在NSCLC细胞中的表达升高主要是由于miR-4299的降低。本研究强调了SLC25A40通过调节细胞增殖和铁凋亡在非小细胞肺癌进展中的关键作用,提示其在非小细胞肺癌的治疗中是一个有希望的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
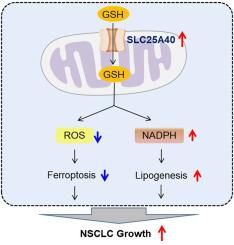
SLC25A40 promotes NSCLC growth by enhancing NADPH-mediated lipid synthesis and suppressing ROS accumulation-induced ferroptosis
Mitochondria serve as vital organelles that play critical roles in regulating cell metabolism and maintaining redox homeostasis. Their dysfunctions are closely associated with the progression of multiple human malignancies. SLC25A40 has been predicted as a mitochondrial carrier required for glutathione import into mitochondria. However, the role of SLC25A40 in human cancers, especially in non-small cell lung cancer (NSCLC), remains poorly understood. Here, we found that SLC25A40 expression was elevated in NSCLC. This upregulation was associated with poor prognosis. Silencing SLC25A40 suppressed NSCLC growth by inhibiting cell proliferation and inducing ferroptosis, whereas its overexpression promoted NSCLC growth. Mechanistically, SLC25A40 promotes cell proliferation by increasing NADPH-mediated lipid synthesis and suppresses ferroptosis by decreasing mitochondrial ROS accumulation. Furthermore, we demonstrated that the elevation of SLC25A40 expression in NSCLC cells was primarily due to decreased miR-4299. This research highlights the pivotal role of SLC25A40 in NSCLC progression by modulating both cell proliferation and ferroptosis, suggesting it as a promising therapeutic target in the management of NSCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


