PERK抑制诱导狼疮性肾炎患者对铁下垂的易感性
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
系统性红斑狼疮(SLE)是一种自身免疫性疾病,儿童最常见、最严重的并发症是狼疮肾炎(LN)。最近的研究发现,铁下垂是LN患者和LN小鼠模型中都存在的病理过程。然而,LN中调节铁下垂的具体分子机制在很大程度上仍未被探索。蛋白激酶rna样内质网激酶(PERK)是一种跨膜蛋白,参与维持细胞稳态和促进细胞存活,在LN的背景下尚未完全表征。我们的实验结果表明,铁下垂标志物在LN患者和狼疮小鼠模型中显著上调。这些变化包括铁离子水平升高,谷胱甘肽(GSH)含量降低,以及死铁相关基因mRNA表达升高。有趣的是,与对照组相比,LN患者和狼疮小鼠的PERK和溶质载体家族7成员11(SLC7A11, xCT) mRNA水平均显著降低。此外,PERK表达与谷胱甘肽水平呈正相关,提示其具有潜在的保护作用。功能研究进一步表明,PERK抑制加重肾损伤和铁下垂,同时降低谷胱甘肽含量。利用HK-2细胞进行的体外实验表明,铁下垂抑制剂Fer-1可以恢复GSH水平,并在PERK敲低的情况下对抗铁下垂。在机制上,PERK通过ATF4正向调节SLC7A11的转录,突出其在维持细胞氧化还原平衡中的作用。综上所述,低PERK表达通过ATF4抑制SLC7A11转录,减少GSH合成,从而加重狼疮肾炎。这些发现为PERK在LN发病机制中的作用提供了新的方向和潜在的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
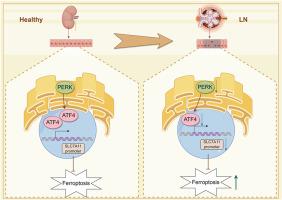
PERK suppression induces susceptibility to ferroptosis in lupus nephritis
Systemic Lupus Erythematosus (SLE) is an autoimmune disease, and the most common and serious complications in children is lupus nephritis (LN). Recent studies have identified ferroptosis as a pathological process present in both LN patients and mouse models of LN. However, the specific molecular mechanisms regulating ferroptosis in LN remain largely unexplored. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) is a transmembrane protein involved in maintaining cellular homeostasis and promoting cell survival, has not been fully characterized in the context of LN. Our experimental findings have demonstrated that ferroptosis markers are significantly upregulated in LN patients and lupus mouse models. These changes include increased Fe2+ levels, decreased glutathione (GSH) content, and elevated mRNA expression of ferroptosis-associated genes. Interestingly, PERK and Solute Carrier Family 7 Member 11(SLC7A11, xCT) mRNA levels were markedly reduced in both LN patients and lupus mice compared to controls. Moreover, PERK expression showed a positive correlation with GSH levels, suggesting a potential protective role. Functional studies further revealed that PERK inhibition exacerbates renal injury and ferroptosis while reducing GSH content. In vitro experiments using HK-2 cells demonstrated that the ferroptosis inhibitor Fer-1 could restore GSH levels and counteract ferroptosis under PERK knockdown conditions. Mechanistically, PERK positively regulates the transcription of SLC7A11 via ATF4, highlighting its role in maintaining cellular redox balance. In conclusion, low PERK expression aggravate lupus nephritis by inhibiting SLC7A11 transcription via ATF4 and reducing GSH synthesis. These findings provide new directions and potential therapeutic targets for the role of PERK in the pathogenesis of LN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


