小胶质源性一氧化氮调节杏仁核突触可塑性,驱动腰椎间盘突出症引起的慢性疼痛和抑郁
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
腰椎间盘突出症(LDH)是慢性腰痛的主要诱因,常伴有抑郁样行为,但将伤害感受与情感障碍联系起来的椎上机制尚不清楚。在这里,我们研究了小胶质细胞来源的一氧化氮(NO)调节大鼠LDH模型杏仁核突触可塑性的潜在机制。行为评估证实LDH大鼠存在机械性痛觉过敏和抑郁样行为。多组学分析显示脑脊液中l -精氨酸增加,杏仁核中cGMP-PKG和谷氨酸能长期增强通路富集。蛋白水平验证证实了杏仁核中iNOS、NO、cGMP和PRKG2的上调。同时,杏仁核和脑脊液中IL-1β和TNF-α水平升高,以及小胶质细胞中Iba1和iNOS共定位,证实了神经炎症微环境。GRIA1、p-GRIA1、GRIN2B和p-CaMKII的表达增强表明杏仁核兴奋性突触传递增强。在小胶质细胞-神经元共培养系统中,来自csf激活的BV2细胞的条件培养基上调PC12细胞的PRKG2、cGMP和突触可塑性标志物。这些作用被iNOS抑制剂1400W消除,并被NO供体delta - nonoate模拟,证实了小胶质NO与神经元可塑性之间的机制联系。这些发现表明,ldl诱导的神经炎症激活杏仁核中的小胶质细胞iNOS,导致NO升高,参与cGMP/PRKG2通路并驱动病理性兴奋性突触可塑性。靶向这一神经免疫通路可能为LDH诱导的慢性疼痛和相关抑郁提供新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
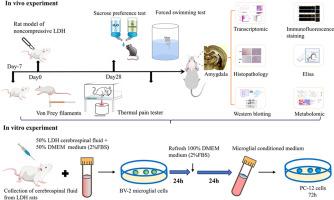
Microglial-derived nitric oxide regulates amygdala synaptic plasticity to drive chronic pain and depression induced by lumbar disc herniation
Lumbar disc herniation (LDH) is a major driver of chronic low back pain often accompanied by depression-like behaviors, yet the supraspinal mechanisms that link nociception to affective disturbance remain unclear. Here, we investigated the potential mechanisms by which microglia-derived nitric oxide (NO) modulates synaptic plasticity in the amygdala of a rat model of LDH. Behavioral assessments confirmed the presence of mechanical hyperalgesia and depression-like behaviors in LDH rats. Multi-omics profiling revealed increased L-arginine in CSF and enrichment of cGMP–PKG and glutamatergic, long-term potentiation pathways in the amygdala. Protein-level validation confirmed upregulation of iNOS, NO, cGMP, and PRKG2 in the amygdala. Concurrently, increased levels of IL-1β and TNF-α in both the amygdala and CSF, along with Iba1 and iNOS co-localization in microglia, confirmed a neuroinflammatory microenvironment. Enhanced expression of GRIA1, p-GRIA1, GRIN2B, and p-CaMKII indicated potentiation of excitatory synaptic transmission in the amygdala. In a microglia-neuron co-culture system, conditioned medium from CSF-activated BV2 cells upregulated PRKG2, cGMP, and synaptic plasticity markers in PC12 cells. These effects were abolished by the iNOS inhibitor 1400W and mimicked by the NO donor DETA-NONOate, confirming a mechanistic link between microglial NO and neuronal plasticity. These findings suggested that LDH-induced neuroinflammation activates microglial iNOS in the amygdala, leading to NO elevations that engage the cGMP/PRKG2 pathway and drive pathological excitatory synaptic plasticity. Targeting this neuroimmune pathway may offer novel therapeutic strategies for chronic pain and related depression induced by LDH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


