冠状病毒刺突HR2结构域:膜融合过程中一个不起眼的参与者?
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
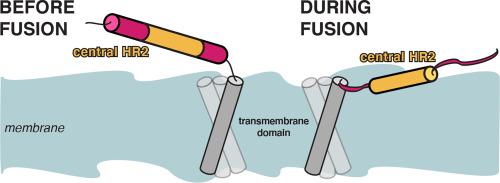
冠状病毒刺突蛋白是实现膜融合的关键实体,它的存在离不开膜活性片段。除了融合肽外,这些结构域还包括HR1和HR2。它们对突刺的再折叠和膜融合至关重要,据信它们相互作用并结合在合并的膜上。为了阐明HR2在这一过程中的确切作用,需要了解其结构和行为。在这里,我们使用了各种计算方法来研究SARS-CoV-2刺突HR2(1163-1211)在活刺突中与膜的相互作用。在模型双分子层的模拟中,HR2对膜的存在仍然没有反应,然而,当扩展到包括跨膜结构域(TMD)(1212-1234)和/或膜活性preHR2片段(1147-1161)时,HR2与模型双分子层的结合明显增强。HR2的三聚体卷曲线圈既不能单独解离,也不能与添加的TMD和/或preHR2分离。分子疏水性电位(MHP)图谱显示,HR2的中心部分具有“教科书”膜活性肽的倾斜斜基序特征,尽管两侧有高度亲水的片段。仅包含该基序的截断的HR2对膜具有更大的亲和力,这表明HR2具有模块化结构,具有被侧翼区域掩盖的膜活性片段,并且可能被HR2邻近结构域和其他因子在刺突被酶切后起作用。这种模块化结构可能已经进化为HR2的膜活性被非常微妙地调节,并在病毒融合的正确时刻精确地“开启”。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The coronavirus spike HR2 domain: An obscure player entering the limelight during membrane fusion?
The coronavirus spike protein, the key entity effectuating membrane fusion, cannot exist without membrane-active fragments. In addition to fusion peptides, among such domains are HR1 and HR2. Crucial to the spike's refolding and membrane fusion, they are believed to both interact with each other and bind to the membranes that are merged. To elucidate HR2's precise role in this process, an understanding of its structure and behaviour is required. Here, we used various computational approaches to study SARS-CoV-2 spike HR2's (1163-1211) interaction with membranes in the context within which it operates in live spike. During simulations with model bilayers, HR2 remained hugely unresponsive to the presence of a membrane, however, when extended to include the transmembrane domain (TMD) (1212-1234) and/or membrane-active preHR2 fragment (1147-1161), HR2’s binding to model bilayers was markedly enhanced. The trimeric coiled-coil of HR2 does not dissociate either on its own or with added TMD and/or preHR2. Molecular hydrophobicity potential (MHP) mapping showed that HR2's central part possesses a tilted oblique-oriented motif characteristic of “textbook” membrane-active peptides, albeit flanked by highly hydrophilic fragments. A truncated HR2 only encompassing this motif had a greater affinity for membranes, suggesting HR2 has a modular structure with a membrane-active segment masked by flanking regions and might be potentiated by HR2-adjacent domains and other factors coming into play after the spike gets enzymatically cleaved. Such a modular structure may have evolved for HR2's membrane activity to be regulated very subtly and “switched on” at precisely the right moment during viral fusion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


