真菌毒素对胎盘功能的影响:重点关注环吡唑酸、脱氧雪腐镰刀菌醇、展霉素和玉米赤霉烯酮的代谢物
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
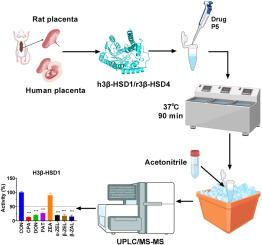
真菌毒素是真菌产生的次生代谢物,对人类和动物健康构成重大风险。本研究旨在测定7种真菌毒素及其代谢物(环吡唑酸、脱氧雪腐烯醇、展霉素、玉米赤霉烯酮、α-玉米赤霉烯醇、β-玉米赤霉烯醇、β-玉米赤霉烯醇、β-玉米赤霉烯醇)对人胎盘3β-羟基类固醇脱氢酶1 (h3β-HSD1)和大鼠同源物r3β-HSD4的抑制效力,以及对人JAr细胞孕酮输出的影响,并进行机制和构效关系分析。在胎盘中,h3β-HSD1负责孕烯醇与孕酮的转化。环吡唑酸、脱氧雪梨烯醇、展曲霉素、α-玉米赤霉烯醇、β-玉米赤霉烯醇和β-玉米赤霉烯醇在100 μM下显著抑制h3β-HSD1活性,而玉米赤霉烯酮则无明显抑制作用。IC50值为8.25 ~ 30.50 μM(展霉素),Ki值为9.04 ~ 31.18 μM,均为混合抑制剂。这些真菌毒素还能抑制≥10 μM JAr细胞的孕酮分泌。对于r3β-HSD4,观察到类似的抑制作用。IC50取值范围为10.55 μM ~ 42.83 μM。真菌毒素与h3β-HSD1和r3β-HSD4的对接分析显示环吡唑酸与NAD+结合位点等相互作用。构效关系分析揭示了分子特性(亲水性、密度)与IC50值之间的相关性。3D-QSAR分析共建立了10个药效团模型,最终选择Hypo1为最佳模型,并通过环吡唑酮、展霉素等化合物进行验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of mycotoxins on placental function: Focus on cyclopiazonic acid, deoxynivalenol, patulin, and zearalenone's metabolites
Mycotoxins are secondary metabolites produced by fungi and pose significant risks to human and animal health. This study aimed to determine inhibitory potency of 7 mycotoxins and metabolites (cyclopiazonic acid, deoxynivalenol, patulin, zearalenone, α-zearalenol, β-zearalenol, and β-zearalanol) against human placental 3β-hydroxysteroid dehydrogenase 1 (h3β-HSD1) and rat homolog r3β-HSD4, as well as their effects on progesterone output in human JAr cells and perform mechanism and structure-activity relationship analysis. In placentas, h3β-HSD1 is responsible for pregnenolone-to-progesterone conversion. Cyclopiazonic acid, deoxynivalenol, patulin, α-zearalenol, β-zearalenol, and β-zearalanol significantly inhibited h3β-HSD1 activity at 100 μM, while zearalenone did not. Their IC50 values ranged from 8.25 μM to 30.50 μM (patulin), and Ki values from 9.04 μM to 31.18 μM, acting as mixed inhibitors. These mycotoxins also inhibited progesterone output by JAr cells at ≥10 μM. For r3β-HSD4, similar inhibitions were observed. IC50 values ranged from 10.55 μM to 42.83 μM. Docking analysis of mycotoxins with h3β-HSD1 and r3β-HSD4 showed interactions like cyclopiazonic acid binding to NAD+-binding site. Structure-activity relation analysis revealed correlations between molecular characteristics (hydrophilicity, density) and IC50 values. 3D-QSAR analysis developed ten pharmacophore models, and Hypo1 was selected as the best model, which was validated by testing compounds like cyclopiazonic and patulin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


