il -11介导的巨噬细胞串扰驱动肾脏炎症和纤维化:慢性肾脏疾病的新治疗靶点
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
目的探讨白细胞介素-11 (IL-11)在慢性肾脏疾病(CKD)进展中的致病作用,并评价IL-11中和抗体在减轻肾脏炎症和纤维化中的治疗潜力。材料和方法我们进行了系统的分子和细胞分析,以表征CKD模型中il -11介导的信号通路。研究了IL-11对巨噬细胞极化、细胞转变和应激反应的影响。临床前疗效研究使用选择性IL-11中和抗体来评估其对肾脏病理的影响。关键发现sil -11通过STAT3信号激活,通过巨噬细胞和小管上皮细胞(tec)之间的免疫串扰协调肾损伤。IL-11促进M1巨噬细胞极化,促进巨噬细胞向肌成纤维细胞转化(MMT),并触发tec中上皮细胞向间充质细胞转化(EMT)。此外,IL-11还能增强氧化和内质网应激反应。用IL-11中和抗体治疗可有效阻断IL-11/IL-11Rα1相互作用和下游STAT3/ erk1 /2介导的超粘附素激活,导致前纤维化细胞外基质沉积减少,TEC再生能力增强,肾实质修复改善。这些发现表明IL-11信号轴是CKD的一个有希望的治疗靶点。基于抗体的干预措施的有效性为CKD治疗提供了一种新的精准医学方法,为肾脏学的临床转化提供了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
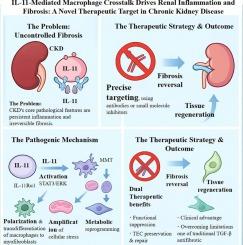
IL-11-mediated macrophage crosstalk drives renal inflammation and fibrosis: A novel therapeutic target in chronic kidney disease
Aims
To investigate the pathogenic role of Interleukin-11 (IL-11) in chronic kidney disease (CKD) progression and evaluate the therapeutic potential of IL-11 neutralizing antibodies in attenuating renal inflammation and fibrosis.
Materials and methods
We conducted systematic molecular and cellular analyses to characterize IL-11-mediated signaling pathways in CKD models. The effects of IL-11 on macrophage polarization, cellular transitions, and stress responses were examined. Preclinical efficacy studies were performed using selective IL-11 neutralizing antibodies to assess their impact on renal pathology.
Key findings
IL-11 orchestrates renal injury through immune crosstalk between macrophages and tubular epithelial cells (TECs) via STAT3 signaling activation. IL-11 promotes M1 macrophage polarization, facilitates the macrophage-to-myofibroblast transition (MMT), and triggers epithelial-to-mesenchymal transition (EMT) in TECs. Additionally, IL-11 amplifies oxidative and endoplasmic reticulum stress responses. Treatment with IL-11 neutralizing antibodies effectively interrupted IL-11/IL-11Rα1 interaction and downstream STAT3/ERK1/2-mediated metadherin activation, resulting in reduced pro-fibrotic extracellular matrix deposition, enhanced TEC regenerative capacity, and improved renal parenchymal repair.
Significance
These findings establish the IL-11 signaling axis as a promising therapeutic target in CKD. The demonstrated efficacy of antibody-based interventions presents a novel precision medicine approach for CKD treatment, offering significant potential for clinical translation in nephrology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


