连续β -颗粒暴露:放射性碘辐照对体外外周血单个核细胞DNA损伤的研究
IF 2.7
3区 医学
Q3 ONCOLOGY
引用次数: 0
摘要
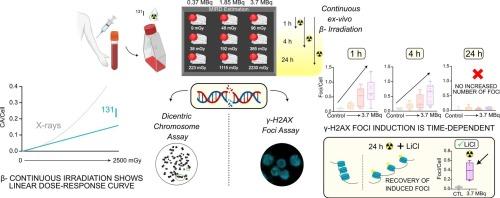
背景与目的靶向放射性核素治疗(TRT)是一种很有前景的癌症治疗方法,但对剂量-反应生物学关系的认识不足限制了其疗效。本研究的目的是利用两种生物标志物:双中心染色体和γ-H2AX焦点,研究暴露于β-连续辐照(131I)的外周血单核细胞(PBMCs)的DNA损伤特征,并评估其与吸收剂量的关系。材料与方法健康供体spbmcs以不同活性(0.37 ~ 3.7 MBq)和时间(1,4,24 h)暴露于131I。吸收剂量采用医学内辐射剂量公式计算。采用染色体畸变频率和γ-H2AX病灶定量评价DNA损伤。用氯化锂(LiCl)评价暴露24 h后对延迟病灶形成的挽救作用。结果连续β-辐照诱导ca呈线性增加(α = 0.0643±0.0068),而急性x线照射诱导ca呈线性二次型增加(α = 0.0359±0.0093,β = 0.0673±0.0042)。与x射线相比,放射性核素照射下ca的频率较低。γ-H2AX在连续照射1和4 h时显著升高,但在连续照射24 h时降低。LiCl处理在24 h时部分恢复了γ-H2AX病灶水平。结论通过ca评价,连续β-辐照下的剂量-反应关系符合线性趋势。DNA损伤引起的病灶表现出随时间变化的动态特性。24小时的聚焦下降,与LiCl可逆,突出了γ-H2AX作为生物标志物在长时间照射条件下的局限性。这些发现强调了优化剂量学方法和确定可靠的TRT生物标志物的必要性。缩写:TRT,靶向放射性核素治疗;医学内辐射剂量;EBRT,外束放疗;LQ,线性二次;双中心染色体测定;DSB,双链断裂;外周血单核细胞;PB,外周血;胎牛血清;PBS,磷酸盐缓冲盐水;CAs,染色体畸变;RT,室温。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Continuous β− particle exposure: A study of DNA damage in ex vivo peripheral blood mononuclear cells irradiation with Radioiodine
Background and purpose
Targeted radionuclide therapy (TRT) is a promising cancer treatment, but insufficient knowledge of the biological dose–response relationships limits its efficacy. The aim of this study was to characterize DNA damage in peripheral blood mononuclear cells (PBMCs) exposed to β-continuous irradiation (131I), and to assess its relationship with the absorbed dose, using two biomarkers: dicentric chromosomes and γ-H2AX foci.
Materials and methods
PBMCs from healthy donors were exposed to 131I at various activities (0.37–3.7 MBq) and times (1, 4, 24 h). Absorbed doses were calculated using the Medical Internal Radiation Dose formalism. DNA damage was assessed by chromosomal aberration frequency and γ-H2AX foci quantification. Lithium chloride (LiCl) was used to evaluate the rescue of delayed foci formation after 24 h exposure.
Results
Continuous β-irradiation induced a linear increase in CAs (α = 0.0643 ± 0.0068), in contrast to the linear-quadratic response observed in acute X-ray exposure (α = 0.0359 ± 0.0093, β = 0.0673 ± 0.0042). The frequency of CAs is lower under radionuclide irradiation compared to X-rays. γ-H2AX increased significantly at 1 and 4 h of continuous exposure but diminished at 24 h, despite continuous irradiation. LiCl treatment partially restored γ-H2AX foci levels at 24 h.
Conclusion
Dose-response relationship under continuous β-irradiation, assessed by CAs, follows a linear trend. DNA damage induced foci show a time-dependent dynamic. The decline in foci at 24 h, reversible with LiCl, highlights the limitations of γ-H2AX as a biomarker under prolonged irradiation conditions. These findings emphasize the need for optimized dosimetry methods and identify reliable biomarkers in TRT.
Abbreviations: TRT, targeted radionuclide therapy; MIRD, Medical Internal Radiation Dose; EBRT, external beam radiotherapy; LQ, linear-quadratic; DCA, dicentric chromosome assay; DSB, double strand break; PBMCs, peripheral blood mononuclear cells; PB, peripheral blood; FBS, fetal bovine serum; PBS, phosphate-buffered saline; CAs, chromosomal aberrations; RT, room temperature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clinical and Translational Radiation Oncology
Medicine-Radiology, Nuclear Medicine and Imaging
CiteScore
5.30
自引率
3.20%
发文量
114
审稿时长
40 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


