氮化锰配体自由基络合物光解活化C-H键
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们报道了溶液稳定的氮化锰配体与自由基配合物[MnV(SalNMe2•)N]+的热活化和光活化,它促进了N - N键的均偶联生成N2,这是MnVI氮化物中常见的反应。理论计算表明,氮化物具有两亲性,有利于N-N键的形成。值得注意的是,MnV配体自由基在室温下的光激活可以快速激活C-H键,导致C-N插入产物的形成,不包括观察到的去饱和产物的二氢蒽。本研究提出了一个罕见的例子,光化学驱动分子间N原子转移到C-H键,由第一排Mn端氮化物络合物介导,这是由于氧化配体自由基电子结构的独特稳定性和开启反应性而成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
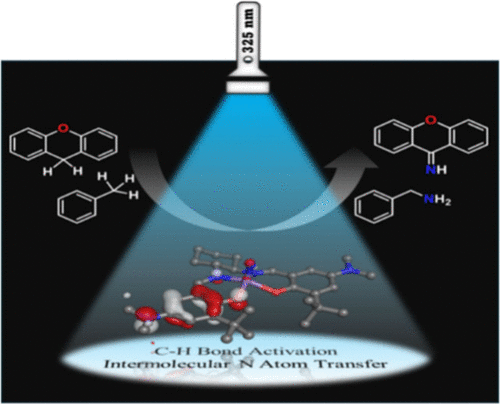
C–H Bond Activation via Photolysis of a Mn Nitride Ligand Radical Complex
We report the thermal and photoactivation of a solution-stable Mn nitride ligand radical complex [MnV(SalNMe2•)N]+, which facilitates N–N bond homocoupling to generate N2, a reaction commonly observed in MnVI nitrides. Theoretical calculations suggest that the nitride is ambiphilic, facilitating the N–N bond formation. Notably, photoactivation of the MnV ligand radical at room temperature enables rapid C–H bond activation, leading to the formation of C–N insertion products, excluding dihydroanthracene where a desaturation product is observed. This study presents a rare example of photochemically driven intermolecular N atom transfer to a C–H bond mediated by a first-row Mn terminal nitride complex, made possible by the unique stability and turn-on reactivity of the oxidized ligand radical electronic structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


