利用低价碱金属掺杂策略提高高熵氧化物催化剂在水气倒转反应中的性能
IF 6.4
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
水气倒转反应(RWGS)是催化CO2增值的重要途径。新兴的高熵氧化物(HEO)体系表现出巨大的催化潜力;然而,他们的活动仍然不够理想。在这项工作中,我们开发了锂掺杂策略来修饰(Mg1Co1Ni1Cu1Zn1)Oα (J14)高熵氧化物(HEO)催化剂,从而提高了RWGS的催化性能。在400°C时,li掺杂催化剂的CO生成速率为210 μmolCO gcat−1 s−1,是J14的1.46倍,稳定性增强。系统表征和实验表明,该方法有效地协调了金属的析出和分散,同时调整了表面碱度,从而增强了H2解离和CO2活化。此外,还观察到从氧化还原途径到碳酸结合途径的机制转变。这种低价碱金属掺杂策略为HEO催化剂提供了一种可推广的设计原则。本文章由计算机程序翻译,如有差异,请以英文原文为准。
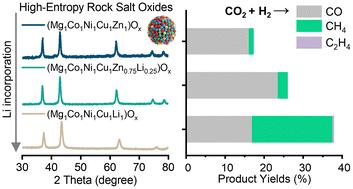
A low-valent alkali metal doping strategy for enhanced performance of high-entropy oxide catalysts in reverse water–gas shift reactions
The reverse water–gas shift (RWGS) reaction is a key pathway for catalytic CO2 valorization. Emerging high-entropy oxide (HEO) systems exhibit great catalytic potential; however, their activity remains suboptimal. In this work, we developed a Li-doping strategy to modify the (Mg1Co1Ni1Cu1Zn1)Oα (J14) high-entropy oxide (HEO) catalyst, yielding enhanced RWGS catalytic performance. The Li-doped catalyst exhibited a CO generation rate of 210 μmolCO gcat−1 s−1 at 400 °C, 1.46 times higher than that of J14, with enhanced stability. Systematic characterization and experiments demonstrated that this approach effectively coordinates metal exsolution and dispersion while tailoring surface alkalinity, thereby enhancing both H2 dissociation and CO2 activation. Furthermore, a mechanistic shift from the redox pathway to the carbonate-associative pathway was observed. This low-valent alkali metal doping strategy offers a generalizable design principle for HEO catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


