电化学选择性氧化醛生成羧酸的催化剂设计
IF 6.4
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在全球碳中和背景下,绿色氢作为清洁能源的关键载体,迫切需要在生产技术上取得突破。在水电解过程中,阳极析氧反应(OER)的高能耗和低效率严重限制了整体能量转换效率。醛类化合物的电催化选择性氧化由于其热力学势低、动力学快,具有取代OER的潜力,并且通过精确的催化剂控制,可以将醛类化合物转化为高值羧酸。本文综述了该工艺的催化剂设计策略,重点介绍了反应机理、设计原则和结构-性能关系。通过对CO键活化和C-H键裂解动力学的分析,提出了基于电子结构调控和协同效应的核心设计原则,并通过原位表征和理论计算建立了催化剂性能模型。这为设计高效催化剂提供了理论指导,并对非贵金属催化剂开发、复杂反应网络调控等未来研究方向进行了展望,为“绿色氢-高值化学品”联产技术的工业应用提供了新思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
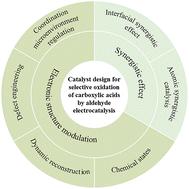
Catalyst design for electrochemical selective oxidation of aldehydes to carboxylic acids
In the global carbon neutrality context, green hydrogen, a key carrier of clean energy, requires urgent breakthroughs in production technology. In water electrolysis, the high energy consumption and low efficiency of the oxygen evolution reaction (OER) at the anode severely limit overall energy conversion efficiency. The electrocatalytic selective oxidation of aldehydes shows potential to replace the OER due to its low thermodynamic potential and fast kinetics, and it can transform aldehydes into high-value carboxylic acids through precise catalyst control. This paper reviews catalyst design strategies for this process, focusing on reaction mechanisms, design principles, and structure–performance relationships. By analyzing the activation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and C–H bond cleavage kinetics, core design principles based on electronic structure regulation and synergistic effects are proposed, and a catalyst performance model is established using in situ characterization and theoretical calculations. This provides theoretical guidance for designing efficient catalysts and prospects for future research directions, such as non-precious metal catalyst development and complex reaction network regulation, offering new ideas for the industrial application of “green hydrogen–high-value chemicals” co-production technology.
O bonds and C–H bond cleavage kinetics, core design principles based on electronic structure regulation and synergistic effects are proposed, and a catalyst performance model is established using in situ characterization and theoretical calculations. This provides theoretical guidance for designing efficient catalysts and prospects for future research directions, such as non-precious metal catalyst development and complex reaction network regulation, offering new ideas for the industrial application of “green hydrogen–high-value chemicals” co-production technology.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and C–H bond cleavage kinetics, core design principles based on electronic structure regulation and synergistic effects are proposed, and a catalyst performance model is established using in situ characterization and theoretical calculations. This provides theoretical guidance for designing efficient catalysts and prospects for future research directions, such as non-precious metal catalyst development and complex reaction network regulation, offering new ideas for the industrial application of “green hydrogen–high-value chemicals” co-production technology.
O bonds and C–H bond cleavage kinetics, core design principles based on electronic structure regulation and synergistic effects are proposed, and a catalyst performance model is established using in situ characterization and theoretical calculations. This provides theoretical guidance for designing efficient catalysts and prospects for future research directions, such as non-precious metal catalyst development and complex reaction network regulation, offering new ideas for the industrial application of “green hydrogen–high-value chemicals” co-production technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


