酵母线粒体大亚基生物发生的后期
IF 3.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2025-08-25
DOI:10.1016/j.bbamcr.2025.120051
引用次数: 0
摘要
酿酒酵母菌线粒体核糖体合成八种线粒体dna编码的氧化磷酸化所必需的蛋白质。线粒体大亚基(mtLSU)的生物发生涉及保守的DEAD-box解旋酶Mrh4和gtpase Mtg1/GTPBP7和Mtg2/GTPBP5。在这里,我们采用了遗传、生化、体外重构和低温电镜等方法来阐明它们在mtLSU组装后期的分层作用。我们发现mrh4介导的bL33m掺入先于Mtg1募集到21S rRNA。在缺乏Mtg1或uL16m的情况下积累的线粒体组装中间体的低温电镜结构显示,Mtg1将21S rRNA H73-75和H93结构域重组到成熟褶皱。这随后使得邻近肽基转移中心区域螺旋的结构和uL6m、uL16m、bL35m和bL36m在mtLSU成熟后期的结合成为可能。出乎意料的是,含有未成熟mtLSU的单体在Mrh4-、bL33m-、uL16m-、Mtg1-和mtg2缺失的线粒体中组装,其水平随着mtLSU颗粒的成熟状态而增加。我们的数据揭示了在组装后期发生的rRNA折叠事件和mrp的结构。他们已经深入了解了装配因子Mrh4、Mtg1和Mtg2在这一过程中的作用,并揭示了线粒体核糖体组装的进化保守机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
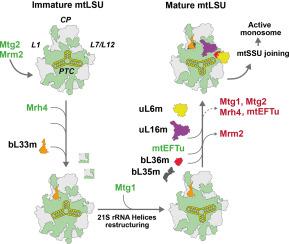
The late stages of yeast mitoribosome large subunit biogenesis
The Saccharomyces cerevisiae mitoribosome synthesizes eight mitochondrial DNA-encoded proteins essential for oxidative phosphorylation. Mitoribosome large subunit (mtLSU) biogenesis involves the conserved DEAD-box helicase Mrh4 and the GTPases Mtg1/GTPBP7 and Mtg2/GTPBP5. Here, we have employed genetic, biochemical, in vitro reconstitution, and cryo-EM approaches to elucidate their hierarchical action during the late stages of mtLSU assembly. We show that Mrh4-mediated bL33m incorporation precedes Mtg1 recruitment to the 21S rRNA. Cryo-EM structures of mitoribosome assembly intermediates accumulating in the absence of Mtg1 or uL16m reveal that Mtg1 restructures the 21S rRNA H73-75 and H93 domains to their mature fold. This subsequently allows the structuring of neighboring peptidyl transfer center region helices and the incorporation of uL6m, uL16m, bL35m, and bL36m during late mtLSU maturation. Unexpectedly, monosomes containing immature mtLSU assemble in Mrh4-, bL33m-, uL16m-, Mtg1-, and Mtg2-depleted mitochondria, at levels that increase with the maturation state of the mtLSU particle. Our data have shed light on the rRNA folding events and the structuring of the MRPs that occur during the late stages of assembly. They have provided insight into the roles of assembly factors Mrh4, Mtg1, and Mtg2 during the process and revealed evolutionarily conserved mechanisms underlying mitochondrial ribosome assembly.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


