剪切应力激活的MMP-2在血管重塑过程中通过LIMK1/Cofilin轴促进骨髓间充质干细胞迁移
IF 2.7
4区 医学
Q2 PERIPHERAL VASCULAR DISEASE
引用次数: 0
摘要
剪切应力增强基质金属蛋白酶-2 (MMP-2)的表达,MMP-2通过与小鼠主动脉内皮细胞(MAECs)的微环境相互作用在骨髓间充质干细胞(BMSCs)迁移和血管重构中起关键作用。使用定制的流动装置将maec暴露于扰动流中1,3或5小时,并收集条件介质(MAEC-CM)。通过流式细胞术、transwell和伤口愈合试验评估骨髓间充质干细胞对不同MAEC-CM条件的迁移。用重组蛋白或中和抗体调节MAEC-CM的MMP-2水平。western blot检测LIMK1/Cofilin通路激活情况,用LIMK1抑制剂BMS-3检测该通路功能。紊乱的血流改变了maec的密度、形态和细胞间隙,随着时间的推移,细胞凋亡增加。ELISA结果显示,MMP-2分泌在3 h达到峰值,与骨髓间充质干细胞最大迁移时间一致。重组MMP-2 (400 ng/mL)进一步增强,而MMP-2中和抗体(100 ng/mL)抑制MAEC-CM-3 h诱导的迁移。Western blot显示,MAEC-CM-3 h后,重组MMP-2处理组的LIMK1和Cofilin磷酸化水平高于中和组。BMS-3显著降低mmp -2诱导的骨髓间充质干细胞迁移和LIMK1/Cofilin磷酸化,但不影响总蛋白水平。这些结果表明,剪切应力诱导的MMP-2通过limk1依赖性的Cofilin激活促进骨髓间充质干细胞的运动。本研究不仅阐明了血流紊乱调节骨髓间充质干细胞迁移的分子机制,也为骨髓间充质干细胞介导的血管修复提供了理论基础,为今后的临床应用提供了潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
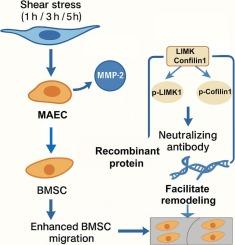
Shear stress-activated MMP-2 promotes BMSCs migration via the LIMK1/Cofilin axis during vascular remodeling
Shear stress enhances matrix metalloproteinase-2 (MMP-2) expression, which plays a critical role in bone marrow mesenchymal stem cells (BMSCs) migration and vascular remodeling via microenvironmental interactions with mouse aortic endothelial cells (MAECs). MAECs were exposed to disturbed flow using a custom flow device for 1, 3, or 5 h, and conditioned media (MAEC-CM) were collected. BMSCs migration in response to different MAEC-CM conditions was assessed by flow cytometry, transwell, and wound-healing assays. MMP-2 levels in MAEC-CM were modulated with recombinant protein or neutralizing antibody. LIMK1/Cofilin pathway activation was evaluated by western blot, and the LIMK1 inhibitor BMS-3 was used to confirm pathway function. Disturbed flow altered MAECs density, morphology, and intercellular gaps, with apoptosis increasing over time. ELISA showed MMP-2 secretion peaked at 3 h, coinciding with maximal BMSCs migration. Recombinant MMP-2 (400 ng/mL) further enhanced, while MMP-2 neutralizing antibody (100 ng/mL) suppressed, migration induced by MAEC-CM-3 h. Western blot revealed significant phosphorylation of LIMK1 and Cofilin after MAEC-CM-3 h treatment, with higher levels in recombinant MMP-2–treated groups compared to neutralization. BMS-3 significantly reduced MMP-2–induced BMSCs migration and phosphorylation of LIMK1/Cofilin without affecting total protein levels. These results indicate that shear stress–induced MMP-2 promotes BMSCs motility through LIMK1-dependent Cofilin activation. This study not only clarifies the molecular mechanism by which disturbed flow regulates BMSCs migration but also provides a theoretical basis for BMSC-mediated vascular repair, offering potential targets for future clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microvascular research
医学-外周血管病
CiteScore
6.00
自引率
3.20%
发文量
158
审稿时长
43 days
期刊介绍:
Microvascular Research is dedicated to the dissemination of fundamental information related to the microvascular field. Full-length articles presenting the results of original research and brief communications are featured.
Research Areas include:
• Angiogenesis
• Biochemistry
• Bioengineering
• Biomathematics
• Biophysics
• Cancer
• Circulatory homeostasis
• Comparative physiology
• Drug delivery
• Neuropharmacology
• Microvascular pathology
• Rheology
• Tissue Engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


