模糊的酰化修饰在心血管疾病中的新作用:还有什么?
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
心血管疾病仍然是全球死亡的主要原因,但其分子机制尚不完全清楚。最近的研究进展强调了一些鲜为人知的酰化修饰在调节心脏稳态和疾病发病机制中的关键作用,如琥珀酰化、巴豆酰化、丙二醛酰化、β-羟基丁基化和乳酸酰化。这些翻译后修饰作为代谢传感器,动态地将细胞代谢与蛋白质的表观遗传和功能变化联系起来。这篇小型综述综合了新出现的证据,揭示了异常的模糊酰化如何通过改变线粒体功能、基因表达或细胞信号传导导致心血管疾病,包括心力衰竭、缺血性损伤和动脉粥样硬化。我们进一步讨论了靶向酰基修饰酶的治疗潜力以及用于修饰预测的机器学习等创新策略。尽管在分析罕见修饰方面存在技术挑战,但该领域为新型生物标志物和精确治疗提供了有希望的途径。通过阐明心血管病理与模糊酰化修饰之间的关系,本综述旨在为心血管疾病的临床干预研究提供启发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
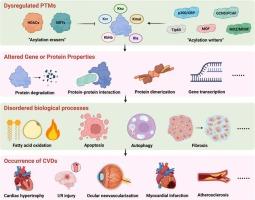
New role of obscure acylation modifications in cardiovascular diseases: what's beyond?
Cardiovascular diseases remain the leading cause of global mortality, yet their molecular mechanisms are incompletely understood. Recent advances highlight the critical role of obscure acylation modifications in regulating cardiac homeostasis and disease pathogenesis, such as succinylation, crotonylation, malonylation, β-hydroxybutyrylation, and lactylation. These post-translational modifications serve as metabolic sensors, dynamically linking cellular metabolism to epigenetic and functional changes in proteins. This mini-review synthesizes emerging evidence on how dysregulated obscure acylations contribute to cardiovascular diseases, including heart failure, ischemic injury, and atherosclerosis, by altering mitochondrial function, gene expression, or cellular signaling. We further discuss the therapeutic potential of targeting acyl-modifying enzymes and innovative strategies like machine learning for modification prediction. Despite technological challenges in profiling rare modifications, this field offers promising avenues for novel biomarkers and precision therapies. By elucidating the relationship between cardiovascular pathologies and obscure acylation modifications, this mini-review aims to inspire future research for clinical intervention of cardiovascular diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


