铜催化叠氮-炔环加成反应中2h -氮嘧啶的反应活性
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
叠氮化物、炔和2H-azirine之间的反应导致在1,2,3-三唑的第5位形成C-C键,而不是先前错误识别的C-N键连接。用标定的QM(DFT-D3)计算充分解释了三唑环上C-C键形成的反应机理。初级产品功能化提供取代嘧啶、呋喃或1,3-恶氮平。本文章由计算机程序翻译,如有差异,请以英文原文为准。
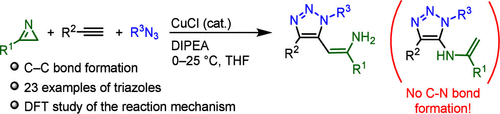
Reactivity of 2H-Azirines in Copper-Catalyzed Azide–Alkyne Cycloaddition Reactions
A reaction between azide, alkyne and 2H-azirine resulted in C–C bond formation at position five of 1,2,3-triazole, instead of previously misidentified C–N bond connectivity. The reaction mechanism of this C–C bond formation on the triazole ring was fully explained by employing calibrated QM(DFT-D3) calculations. Functionalization of primary products provided substituted pyrimidine, furan or 1,3-oxazepine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


