ATF6激活改变结肠脂质代谢导致肿瘤相关微生物适应
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
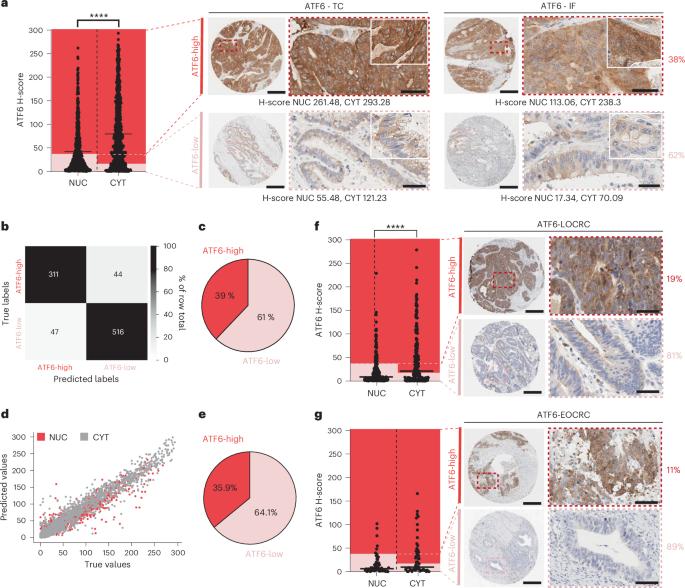
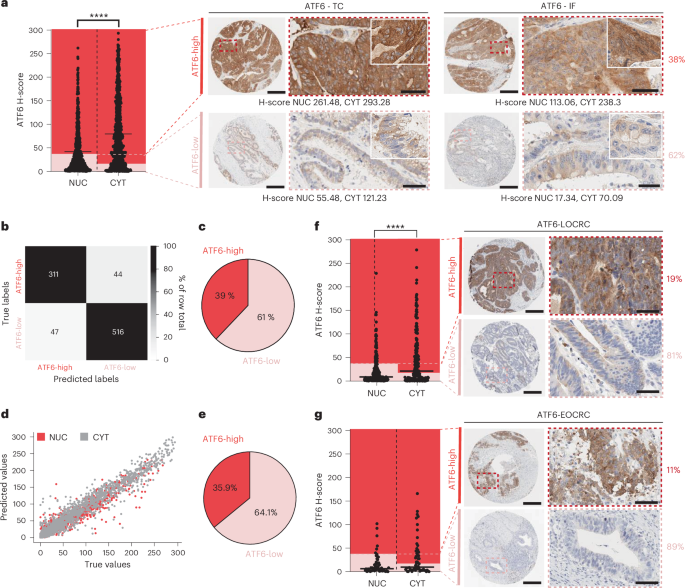
内质网未折叠蛋白反应有助于癌症的发展,激活转录因子6 (ATF6)参与微生物依赖的肿瘤发生。在这里,我们展示了ATF6在早发性和晚期结直肠癌患者中的临床相关性,并将ATF6信号传导与脂质代谢和肠道微生物群的变化联系起来。对ATF6转基因小鼠肠上皮细胞(nATF6IEC)的转录分析发现了细菌特异性的细胞代谢变化,这些细胞代谢丰富了脂肪酸的生物合成。非靶向代谢组学和同型标记证实,atf6相关的长链脂肪酸在人类、小鼠和类器官的结肠组织中富集。在无菌的nATF6IEC小鼠中,FASN抑制和微生物群转移证实了atf6诱导的脂质改变在肿瘤发生中的因果关系。利用生物正交非规范氨基酸标记和分离的Desulfovibrio生长分析,研究了肿瘤相关微生物类群(包括Desulfovibrio fairfield densis)的选择性扩增与长链脂肪酸暴露的机制联系。我们假设慢性ATF6信号通过改变脂质代谢来选择促进肿瘤的微生物群。本文章由计算机程序翻译,如有差异,请以英文原文为准。
ATF6 activation alters colonic lipid metabolism causing tumour-associated microbial adaptation
Endoplasmic reticulum unfolded protein responses contribute to cancer development, with activating transcription factor 6 (ATF6) involved in microbiota-dependent tumorigenesis. Here we show the clinical relevance of ATF6 in individuals with early-onset and late colorectal cancer, and link ATF6 signalling to changes in lipid metabolism and intestinal microbiota. Transcriptional analysis in intestinal epithelial cells of ATF6 transgenic mice (nATF6IEC) identifies bacteria-specific changes in cellular metabolism enriched for fatty acid biosynthesis. Untargeted metabolomics and isotype labelling confirm ATF6-related enrichment of long-chain fatty acids in colonic tissue of humans, mice and organoids. FASN inhibition and microbiota transfer in germ-free nATF6IEC mice confirm the causal involvement of ATF6-induced lipid alterations in tumorigenesis. The selective expansion of tumour-relevant microbial taxa, including Desulfovibrio fairfieldensis, is mechanistically linked to long-chain fatty acid exposure using bioorthogonal non-canonical amino acid tagging, and growth analysis of Desulfovibrio isolates. We postulate chronic ATF6 signalling to select for tumour-promoting microbiota by altering lipid metabolism. The transcription factor ATF6 causes an enrichment in long-chain fatty acids in the colonic epithelium, which leads to changes in the gut microbiota and contributes to the development of colorectal cancer in humans and mice, thereby linking endoplasmic reticulum stress responses to lipid metabolism and tumorigenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


