下调MiD49和MiD51可减轻胶原诱导的关节炎,抑制类风湿关节炎成纤维细胞样滑膜细胞的线粒体自噬和脂肪酸氧化(FAO)
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
越来越多的证据证实,线粒体动力学失衡会损害线粒体功能,从而破坏细胞内稳态,并可能导致多种疾病。本研究探讨49和51 kDa线粒体动力学蛋白(MiD49和MiD51, MiDs)是否有助于维持成纤维细胞样滑膜细胞(FLS)的异常功能,从而参与类风湿关节炎(RA)的病理过程,并阐明其具体机制。我们发现,在RA患者和胶原诱导关节炎(CIA)模型的滑膜组织FLS以及RA患者的血清中,MiDs显著上调。RA血清MiDs表达升高与临床指标呈正相关。此外,敲低MiD49或MiD51可减轻CIA症状并减轻RA-FLS的攻击行为。我们发现,通过蛋白相互作用(PPI)分析,揭示了MiDs与pten诱导的激酶1 (PINK1)- park2e3泛素蛋白连接酶(Parkin)通路之间的潜在相互作用,以及PINK1-Parkin通路与脂质代谢之间的相关性。在sirna介导的MiD49或MiD51敲低后,pink1 - parkin依赖性线粒体自噬和肉碱棕榈酰基转移酶- 1a (CPT-1A)介导的脂肪酸β氧化(FAO)受损。我们发现,sirna介导的PINK1和Parkin的下调有效地逆转了RA-FLS的侵袭性表型。最后,我们进一步验证了靶向MiD49或MiD51的shRNA在CIA模型滑膜组织衍生的FLS中抑制了pink1 - parkinson依赖性线粒体自噬和cpt - 1a调控的FAO。我们的研究强调了mids介导的线粒体动力学功能障碍的参与有助于维持FLS的侵袭性,从而参与RA的发病机制。这些发现为今后开发RA的潜在治疗方法提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
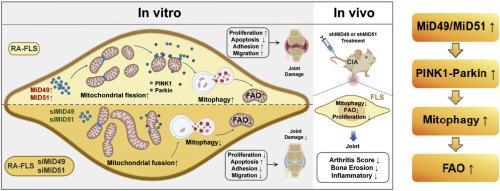
Knockdown of MiD49 and MiD51 alleviates collagen-induced arthritis and suppresses mitophagy and fatty acid oxidation (FAO) in rheumatoid arthritis fibroblast-like synoviocytes
Increasing evidence confirms that imbalances in mitochondrial dynamics can impair mitochondrial function, thereby disrupting cellular homeostasis and potentially contributing to a variety of diseases. This study investigated whether mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51, MiDs) contribute to the maintenance of the abnormal functions of fibroblast-like synoviocytes (FLS), thereby participating in the pathological process of rheumatoid arthritis (RA), and to elucidate the specific mechanisms. We found that MiDs were significantly upregulated in the FLS of synovial tissues from RA patients and collagen-induced arthritis (CIA) models, as well as in the serum of RA patients. The elevated expression of MiDs in RA serum exhibited a positive correlation with clinical markers. Moreover, knocking down MiD49 or MiD51 alleviated CIA symptoms and attenuated the aggressive behavior of RA-FLS. We found the potential interactions between MiDs and the PTEN-induced kinase 1 (PINK1)-PARK2 E3 ubiquitin-protein ligase (Parkin) pathway, as well as the correlation between the PINK1-Parkin pathway and lipid metabolism, were revealed through protein-protein interaction (PPI) analysis. The PINK1-Parkin-dependent mitophagy and carnitine palmitoyltransferase-1A (CPT-1A) mediated-fatty acid β oxidation (FAO) were impaired following siRNA-mediated knockdown of MiD49 or MiD51. We found that siRNA-mediated knockdown of PINK1 and Parkin effectively reversed the aggressive phenotype of RA-FLS. Finally, we further verified that shRNA targeting MiD49 or MiD51 inhibited Pink1-Parkin-dependent mitophagy and CPT-1A-regulated FAO in FLS derived from the synovial tissues of CIA models. Our study highlights the involvement of MiDs-mediated mitochondrial dynamics dysfunction helps maintain the invasiveness of FLS, and thereby participates in the pathogenesis of RA. These findings provide a theoretical basis for the development of potential therapies for RA in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


