美托洛尔加重东莨菪碱诱导的认知障碍大鼠痴呆:NADPH氧化酶的潜在作用
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
β受体阻滞剂与认知障碍有关,一些研究表明它们会增加血管性痴呆(VD)的风险。虽然先前的临床和临床前研究已经将β受体阻滞剂(包括美托洛尔)与认知能力下降联系起来,但分子机制仍不清楚。本研究旨在阐明美托洛尔对东莨菪碱诱导的大鼠认知功能障碍的影响,重点关注NADPH氧化酶介导的氧化应激的作用。成年雄性Wistar大鼠单用美托洛尔(30 mg/kg/d,口服)或联合东莨菪碱(1 mg/kg/d,口服)。为了评估NADPH氧化酶的作用,一组大鼠还接受了一种特异性NADPH氧化酶抑制剂罗布麻苷(10mg /kg/day; i.p)。行为测试用于评估认知功能,生化分析用于测量氧化应激标志物、神经炎症介质和线粒体生物发生相关蛋白。美托洛尔加重了东莨菪碱引起的认知能力下降,其表现为学习和记忆能力受损。这种效果伴随着海马NADPH氧化酶活性、氧化应激生物标志物和p38 MAPK/NF-κ b介导的神经炎症的增加。随后,美托洛尔通过负调控SIRT1/PGC-1α/NRF1/TFAM信号轴破坏线粒体生物发生机制,导致细胞凋亡。同时服用罗布麻甙可逆转大部分这些改变,其中氧化应激、神经炎症和线粒体功能障碍的减弱被确定。综上所述,美托洛尔可能通过氧化应激放大、神经炎症和线粒体生物发生损伤显著加重认知障碍。这些发现表明,在给老年患者开美托洛尔处方时需要谨慎,尤其是那些有认知能力下降风险的患者。靶向NADPH氧化酶可能提供潜在的治疗方法来抵消美托洛尔对认知功能的不良影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
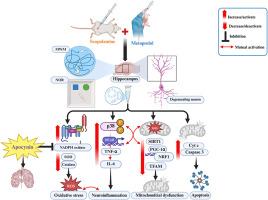
Metoprolol exacerbates dementia in scopolamine-induced cognitive impairment in rats: A potential role of NADPH oxidase
β-blockers have been implicated in cognitive impairment, with some studies suggesting their role in increasing the risk of vascular dementia (VD). While previous clinical and preclinical research has linked β-blockers, including metoprolol, to cognitive decline, the molecular mechanisms remain unclear. This study aims to elucidate the impact of metoprolol on scopolamine-induced cognitive impairment in rats, focusing on the role of NADPH oxidase-mediated oxidative stress. Adult male Wistar rats were administered metoprolol (30 mg/kg/day; p.o) alone or in combination with scopolamine (1 mg/kg/day; i.p). To assess the involvement of NADPH oxidase, a subset of rats also received apocynin (10 mg/kg/day; i.p), a specific NADPH oxidase inhibitor. Behavioral tests were performed to evaluate cognitive function, while biochemical analyses were conducted to measure oxidative stress markers, neuroinflammatory mediators, and mitochondrial biogenesis-related proteins. Metoprolol exacerbated scopolamine-induced cognitive decline, which was unvieled through the impaired learning and memory performance. This effect was accompanied by increased hippocampal NADPH oxidase activity, oxidative stress biomarkers, and p38 MAPK/NF-κB-mediated neuroinflammation. Subsequently, metoprolol disrupted mitochondrial biogenesis machinery through the negative regulation of the SIRT1/PGC-1α/NRF1/TFAM signaling axis, which was followed by apoptotic cell death. Co-administration of apocynin reversed most of these alterations, where attenuation of oxidative stress, neuroinflammation, and mitochondrial dysfunction were identified. In conclusion, metoprolol significantly worsens cognitive impairment, likely through oxidative stress amplification, neuroinflammation, and mitochondrial biogenesis impairment. These findings suggest that caution is needed when prescribing metoprolol to elderly patients, especially those at risk of cognitive decline. Targeting NADPH oxidase may offer a potential therapeutic approach to counteract metoprolol's adverse effects on cognitive function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


