噻唑烷酮类及相关类似物作为有效的抗锥虫药物
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
设计并合成了一系列噻唑烷二酮及其类似物,并对其抗寄生虫活性进行了评价。一项结构-活性关系(SAR)研究聚焦于分子特定部分的修饰,揭示了对布鲁氏锥虫具有显著活性的衍生物。值得注意的是,类似物6i对引起人类非洲锥虫病的原生动物布鲁氏罗得西亚锥虫具有特殊的活性,EC50值为30 nM,选择性指数为2000。此外,化合物6a、6k、7e和18在小于5 μM范围内具有抗锥虫活性。我们最活跃的类似物6i代表了进一步临床前开发的有希望的候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
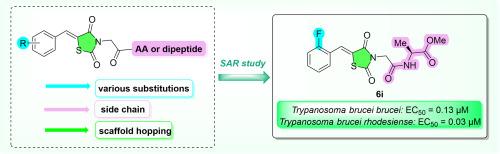
Thiazolidinones and related analogues as efficient antitrypanosomal agents
We designed and synthesised a series of thiazolidinediones and related analogues and evaluated their antiparasitic activity. A structure-activity relationship (SAR) study focused on modifications of specific parts of the molecule revealed derivatives that displayed significant activity against Trypanosoma brucei species. Notably, the analogue 6i exhibited exceptional activity, with an EC50 value of 30 nM and a selectivity index of >2000, against the protozoan Trypanosoma brucei rhodesiense, which causes human African trypanosomiasis. Additionally, compounds 6a, 6k, 7e, and 18 demonstrated antitrypanosomal activities in the less than 5 μM range. Our most active analogue 6i represents a promising candidate for further preclinical development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


