HDAC双抑制剂在乳腺癌治疗中的研究进展
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
乳腺癌仍然是全球妇女中最常见的恶性肿瘤,是癌症相关死亡的主要原因。虽然治疗取得了进步,但在治疗耐药性、疾病复发和远处转移方面的持续挑战继续破坏临床结果。组蛋白去乙酰化酶(hdac)是一个保守的表观遗传调控家族,它催化组蛋白底物乙酰基的去除,从而协调染色质重塑和转录调控。除了其典型的表观遗传功能外,这些酶还对多种致癌过程进行关键调节,包括细胞增殖、分化和转移,使其成为肿瘤学中有希望的治疗靶点。最近的研究已经证明了双靶点抑制剂的治疗前景。目前的证据表明,这种联合方法不仅可以通过多模式机制增强抗肿瘤疗效,而且可以避免单靶点治疗中常见的耐药现象。这种治疗模式的转变强调了以hdac为基础的双抑制剂治疗乳腺癌的临床潜力。在这篇综述中,我们系统地分析了双靶点HDAC抑制剂(HDACis)的最新进展,整合了机制见解、临床前证据和转化意义,为未来的治疗开发和临床实施建立了基础框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
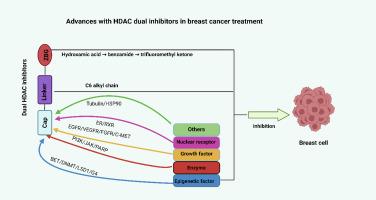
Advances of HDAC dual inhibitors in breast cancer treatment
Breast cancer remains the most common prevalent malignancy among women globally, constituting the primary cause of cancer-associated mortality. While therapeutic advancements have been achieved, persistent challenges in treatment resistance, disease recurrence, and distant metastasis continue to undermine clinical outcomes. Histone deacetylases (HDACs), a conserved family of epigenetic regulators, catalyze the removal of acetyl groups from histone substrates, thereby orchestrating chromatin remodeling and transcriptional regulation. Beyond their canonical epigenetic functions, these enzymes critically modulate diverse oncogenic progresses, including cell proliferation, differentiation, and metastasis, positioning them as promising therapeutic targets in oncology. Recent studies have demonstrated the therapeutic prospects of dual-target inhibitors. Current evidence suggests such combinatorial approaches not only enhance anti-neoplastic efficacy through multi-modal mechanisms but also circumvent the drug resistance frequently observed in single-target therapy. This therapeutic paradigm shift underscores the clinical potential of HDAC-based dual inhibitors for breast cancer management. In this review, we systematically analyze recent advancements in dual-target HDAC inhibitors (HDACis), integrating mechanistic insights, preclinical evidences, and translational implications to establish a foundational framework for future therapeutic development and clinical implementation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


