在水介质中,贱金属介导烷基/芳基/异芳基甲基卤化物直接合成醛和二醛的无酸一锅法
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在这里,我们展示了三种新的贱金属催化剂系统的第一个例子,它们有效地将芳基和烷基甲基卤化物直接转化为醛。所开发的催化剂体系采用廉价和市售的贱金属配合物即醋酸镍、醋酸亚铁和醋酸铁作为催化剂,以六亚甲基四胺为试剂,非常经济。与传统方法不同,开发的合成策略i)避免使用化学计量量的酸,并使用非常便宜的贱金属催化剂,ii)通过避免分离取代的六亚甲基四胺,一步生成醛产品,iii)在纯水介质中工作,iv)发现不仅对芳基醛的合成有效,而且对具有挑战性的脂肪醛,杂芳香醛以及二醛。v)甚至从更便宜的甲基氯化合物开始生产醛产品,从而进一步降低了该过程的成本。对所有催化剂体系的可回收性进行了测试,发现系统在第七次循环之前都相当活跃。DFT研究表明,在HMTA与金属中心配位后,HOMO-LUMO间隙显著减小。在所有考虑用于DFT研究的配合物中,Ni(OAc)2。HMTA具有最小的HOMO-LUMO间隙,这反映在它的催化活性上,使得其醛产物的产率高于其他催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
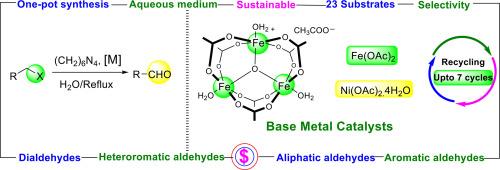
Base metal mediated acid-free one pot synthesis of aldehydes and dialdehydes directly from alkyl/aryl/heteroaryl-methyl halides in aqueous medium
Herein, we demonstrated the first examples of three new base metal catalyst systems that efficiently convert the aryl and alkyl methyl halides directly into aldehydes. The developed catalyst systems are very economical as they employ the cheap and commercially available base metal complexes namely, nickel acetate, ferrous acetate and ferric acetate as catalysts and hexamethylenetetramine as the reagent. Unlike the conventional methods the developed synthetic strategies i) avoid the use of stoichiometric amount of acids and employs very cheap base metal catalysts, ii) yield the aldehyde products in a single step by avoiding isolation of substituted hexamethylenetetramine, iii) work in pure aqueous medium iv) found to be active not only for the synthesis of aryl aldehydes but also for the challenging aliphatic aldehydes, heteroaromatic aldehydes as well as dialdehydes, and v) yield the aldehyde products even from starting from cheaper methyl chloro compounds thus reducing the cost of the process further. The recyclability of all the catalyst systems was tested and found that the systems were considerably active till the seventh cycle. DFT studies revealed that a considerable reduction in the HOMO-LUMO gap was observed upon coordination of HMTA to the metal centre. Among all the complexes considered for DFT studies, Ni(OAc)2.HMTA had the lowest HOMO-LUMO gap and the same was reflected in its catalytic activity, which gave the aldehyde product in highest yield in comparison to other catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


