水凝胶粘接剂与水动力诱导的液-固过渡用于微椎间盘切除术后的环裂密封和炎症调节
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
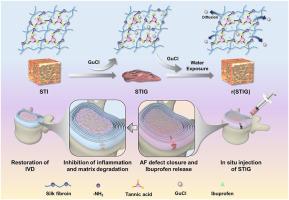
背景:椎间盘(IVD)突出是腰痛和残疾的主要原因,微椎间盘切除术是标准的手术治疗。然而,微椎间盘切除术不能解决纤维环(AF)缺陷,增加了复发性疝的风险。目前的治疗策略对这种情况的疗效仍然有限。损伤后缺乏修复和未解决的炎症可进一步损害IVD功能,最终导致不可逆的IVD变性。因此,开发一种既能机械稳定环裂又能局部抗炎药物递送的房颤粘接剂是解决这一临床挑战的有希望的策略。方法以丝素、单宁酸、布洛芬、盐酸胍(GuCl)为主要原料制备AF胶粘剂体系STIG。对STIG进行了综合评价,包括其微观结构、组成、可注射性、组织粘附性、流变性能和生物相容性。为了评估抗炎效果,通过脂多糖(LPS)刺激的AF细胞建立了体外炎症微环境。为了在体内验证,通过穿刺AF来模拟髓核(NP)疝,通过手术诱导大鼠IVD变性模型。该实验框架能够评估STIG预防NP突出、调节炎症反应和延迟IVD变性的能力。结果在STIG系统中,GuCl起到氢键干扰物的作用,促进其释放到体液中,从而允许氢键的重组。这种特性使STIG能够在与水接触时从可注射的低刚度状态转变为高刚度的粘合凝胶。在粘合剂中加入布洛芬可以有效地抑制炎症介质的产生和细胞外基质成分的分解。在大鼠尾模型中,STIG有效地保留了NP含水量,维持了椎间盘高度指数,保障了IVD术后的结构完整性。结论:STIG作为一种治疗房颤裂和预防房颤退行性变的有效方法。这篇文章的翻译潜力在脊柱外科中显示了显著的临床潜力。它为减少微椎间盘切除术后的复发率和改善患者的长期预后提供了一种新的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogel adhesives with a hydrodynamically induced liquid–solid transition for annular fissure sealing and inflammation modulation following microdiscectomy
Background
Intervertebral disc (IVD) herniation is a major cause of low back pain and disability, with microdiscectomy being the standard surgical treatment. However, microdiscectomy fails to address annulus fibrosus (AF) defects, increasing the risk of recurrent herniation. Current therapeutic strategies for this condition remain limited in efficacy. The lack of repair following injury and unresolved inflammation can further damage the IVD function, ultimately leading to irreversible IVD degeneration. Therefore, the development of an AF adhesive capable of both mechanically stabilizing annular fissures and enabling localized anti-inflammatory drug delivery emerges as a promising strategy to address this clinical challenge.
Methods
The developed AF adhesive system, designated as STIG, is formulated from silk fibroin, tannic acid, ibuprofen, and guanidine hydrochloride (GuCl). A comprehensive evaluation is conducted on STIG, encompassing its microstructure, composition, injectability, tissue adhesion, rheological properties, and biocompatibility. To assess anti-inflammatory efficacy, an in vitro inflammatory microenvironment is established via lipopolysaccharide (LPS)-stimulated AF cells. For in vivo validation, a rat model of IVD degeneration is surgically induced through puncturing the AF to simulate nucleus pulposus (NP) herniation. This experimental framework enables evaluation of STIG's ability to prevent NP protrusion, modulate inflammatory responses, and delay IVD degeneration.
Results
In the STIG system, GuCl serves the role of a hydrogen bond disruptor, facilitating its release into bodily fluids, which in turn allows for the reformation of hydrogen bonds. This property endows STIG with the ability to transition from an injectable, low-stiffness state to a high-stiffness adhesive gel upon contact with water. The inclusion of ibuprofen in the adhesive effectively curbs the production of inflammatory mediators and the breakdown of extracellular matrix constituents. In a rat tail model, STIG effectively preserves the NP water content, maintains the disc height index, and safeguards the structural integrity of the IVD post-surgery.
Conclusion
These findings highlight STIG's potential as a promising therapeutic solution for sealing AF fissures and preventing IVD degeneration.
The translational potential of this article
STIG shows significant clinical potential in spinal surgery. It offers a novel approach to reduce the recurrence rate post-microdiscectomy and improving long-term patient outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


