米氮平在早期肿瘤转化中的肝脏免疫微环境重编程:来自二乙基亚硝胺诱导的大鼠模型的证据
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肝肿瘤转化是一个由慢性炎症、氧化应激、免疫逃避和细胞增殖失调驱动的多步骤过程。阻断这些早期事件可能为预防肝细胞癌提供一种可行的策略。在这项研究中,我们研究了米氮平(一种fda批准的抗抑郁药,已知具有抗炎和抗氧化特性)在二乙基亚硝胺诱导的早期肝肿瘤大鼠模型中的化学预防潜力。米氮平显著减轻肝毒性,降低血清转氨酶,氧化应激标志物,恢复抗氧化防御。组织病理学评估显示,米氮平显著降低了灶性肝细胞改变(FAH)、核异型性和炎症浸润的发生率。我们的研究结果显示,CD4+和CD8+ T细胞的浸润和组织水平增强,Th1/Th17细胞因子(IFN-γ、IL-2、IL-12、IL-17)和趋化因子CXCL10上调,免疫抑制介质(IL-10、TGF-β、sCD163)受到抑制。此外,米氮平下调促炎细胞因子IL-6和TNF-α,促进巨噬细胞募集(CD68),并将极化转向m1样表型,iNOS表达增加证明了这一点。米氮平还抑制促增殖和血管生成标志物(Ki67, Cyclin D1, VEGF, MMP-2),重新激活凋亡通路(Bax, caspase-3, p53),抑制致癌STAT3和ERK1/2信号传导。相关性和疾病预测分析表明,免疫激活标记物是FAH负担的强烈阴性预测因子。重要的是,系统生物学和网络分析证实了这些发现,证明米氮平调节关键的免疫调节网络,特别是那些控制T细胞反应、细胞因子信号传导和巨噬细胞极化的网络,从而从机制上验证了其免疫调节和抗肿瘤作用。这些结果强调了米氮平在炎症驱动的肝肿瘤中作为化学预防剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
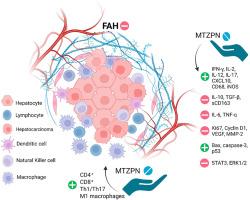
Reprogramming the hepatic immune microenvironment with mirtazapine in early neoplastic transformation: Evidence from a diethylnitrosamine-induced rat model
Hepatic neoplastic transformation is a multistep process driven by chronic inflammation, oxidative stress, immune evasion, and dysregulated cell proliferation. Intercepting these early events may offer a viable strategy for preventing hepatocellular carcinoma. In this study, we investigated the chemopreventive potential of mirtazapine, an FDA-approved antidepressant with known anti-inflammatory and antioxidant properties, in a diethylnitrosamine-induced rat model of early hepatic neoplasia. Mirtazapine significantly attenuated hepatotoxicity, reducing serum transaminases, oxidative stress markers, and restoring antioxidant defenses. Histopathological evaluation revealed that mirtazapine markedly reduced the incidence of foci of altered hepatocytes (FAH), nuclear atypia, and inflammatory infiltration. Our results showed enhanced infiltration and tissue levels of CD4+ and CD8+ T cells, along with upregulation of Th1/Th17 cytokines (IFN-γ, IL-2, IL-12, IL-17) and the chemokine CXCL10, alongside suppression of immunosuppressive mediators (IL-10, TGF-β, sCD163). Additionally, mirtazapine downregulated pro-inflammatory cytokines IL-6 and TNF-α, promoted macrophage recruitment (CD68), and shifted polarization toward an M1-like phenotype, as evidenced by increased iNOS expression. Mirtazapine also inhibited pro-proliferative and angiogenic markers (Ki67, Cyclin D1, VEGF, MMP-2), reactivated apoptotic pathways (Bax, caspase-3, p53), and suppressed oncogenic STAT3 and ERK1/2 signaling. Correlation and disease prediction analyses identified immune activation markers as strong negative predictors of FAH burden. Importantly, systems biology and network analysis confirmed these findings by demonstrating that mirtazapine modulates key immune-regulatory networks, particularly those governing T cell responses, cytokine signaling, and macrophage polarization, thus mechanistically validating its immunomodulatory and anti-tumor effects. These results highlight mirtazapine's potential for repurposing as a chemopreventive agent in inflammation-driven hepatic neoplasia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


