胰腺导管内乳头状肿瘤的多组学分析揭示了不同的模式和潜在的进展标记
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
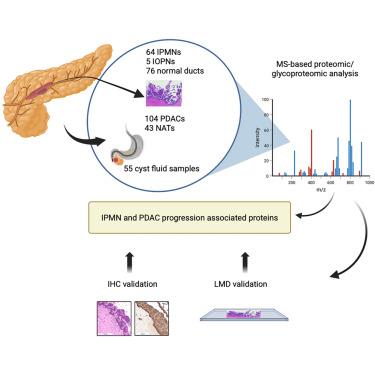
为了能够从癌前病变中早期发现胰腺癌,我们使用质谱分析了64例导管内乳头状粘液瘤(IPMNs)、55例囊肿液样本、104例胰腺导管腺癌(PDACs)和各种类型的正常样本的蛋白质和糖蛋白。与低级别病变相比,高级别IPMNs表现出糖基化水平和肿瘤进展途径的富集。高级别IPMN相关蛋白,如PLOD3、IRS2、LGALS9和Trop-2,通过免疫标记和激光显微解剖鉴定和验证。在胰腺囊肿液中也检测到一些高级别相关蛋白,这使我们能够将肿瘤细胞中表达的蛋白和糖蛋白与临床可获得的生物标本联系起来。与正常导管相比,IPMNs细胞外基质(ECM)蛋白的糖基化水平发生了改变。此外,我们发现ipmn的一个子集具有pdac样特征,包括ECM蛋白的表达升高。这些发现提供了对进展相关蛋白的深入了解,并强调了这些蛋白在胰腺肿瘤中的诊断和治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multi-omic profiling of intraductal papillary neoplasms of the pancreas reveals distinct patterns and potential markers of progression
To enable early detection of pancreatic cancer from precancerous lesions, we analyze proteins and glycoproteins from 64 intraductal papillary mucinous neoplasms (IPMNs), 55 cyst fluid samples, 104 pancreatic ductal adenocarcinomas (PDACs), and various types of normal samples using mass spectrometry. High-grade IPMNs show enrichment of glycosylation level and tumor progression pathways compared to low-grade lesions. High-grade IPMN associated proteins, such as PLOD3, IRS2, LGALS9, and Trop-2, are identified and validated using immunolabeling and laser microdissection. Some high-grade associated proteins are also detected in pancreatic cyst fluids, which allows us to link proteins and glycoproteins expressed in neoplastic cells to clinically accessible biospecimens. Altered glycosylation level of extracellular matrix (ECM) proteins is observed in IPMNs compared to normal ducts. Additionally, we identify a subset of IPMNs with PDAC-like features, including elevated expression of ECM proteins. These findings offer insight into progression-associated proteins and emphasize the diagnostic and therapeutic potential of these proteins in pancreatic tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


